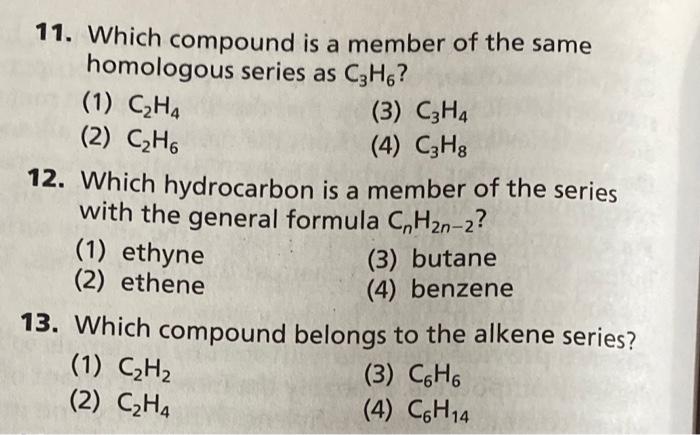Hydrocarbons. ppt download
The hydrocarbon c2h4 is hot sale a member of the series.
Share.
Visit »

Lesson Explainer Homologous Series Nagwa 
Alkenes Homologous series National 5 Chemistry Revision BBC 
Ethylene Wikipedia 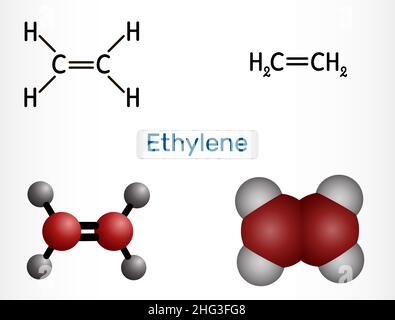
Ethylene ethane C2H4 molecule. It is organic compound 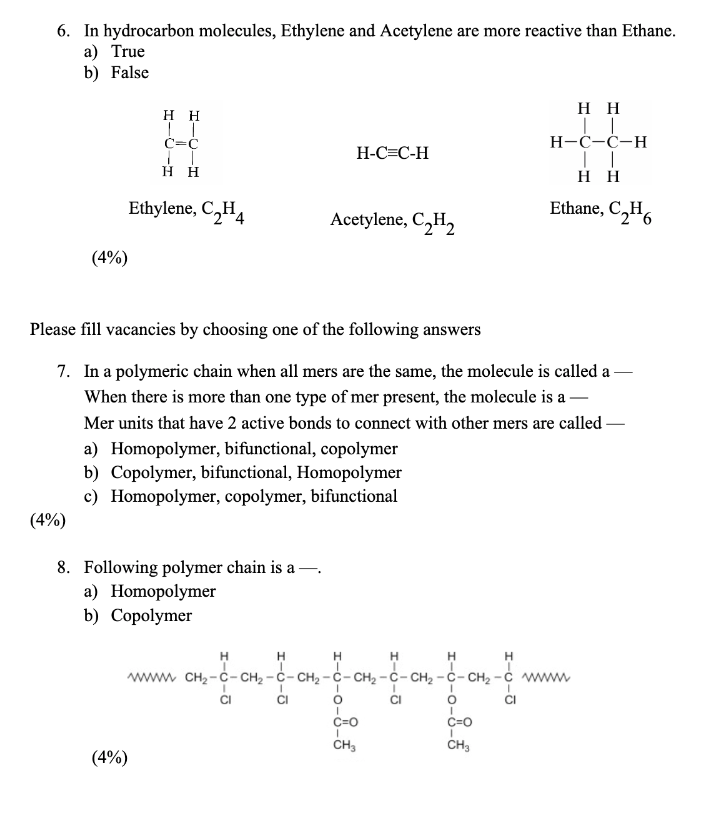
Solved 6. In hydrocarbon molecules Ethylene and Acetylene Chegg 
Chemistry Hydrocarbons Mrs. Ferren Flashcards Quizlet 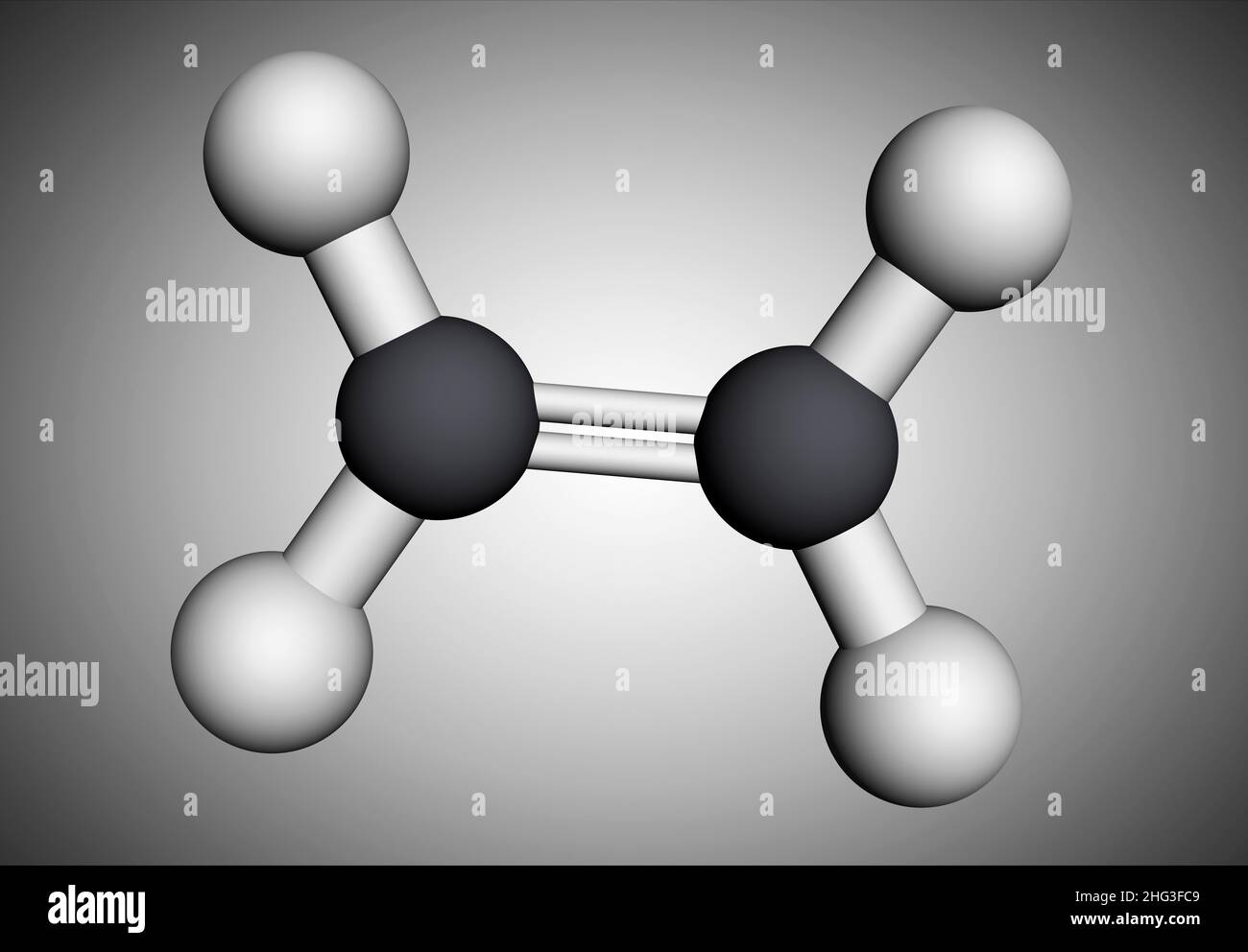
Ethylene ethane C2H4 molecule. It is organic compound 
C15 hydrocarbons PPT 
How is the structural formula for C2H4 determined Quora 
PPT Type of Hydrocarbon PowerPoint Presentation free download 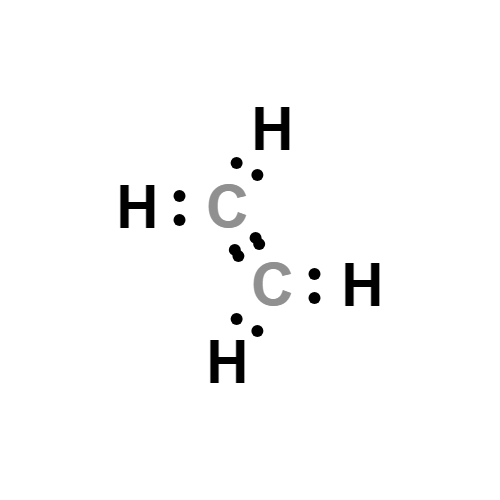
ETHYLENE 74 85 1 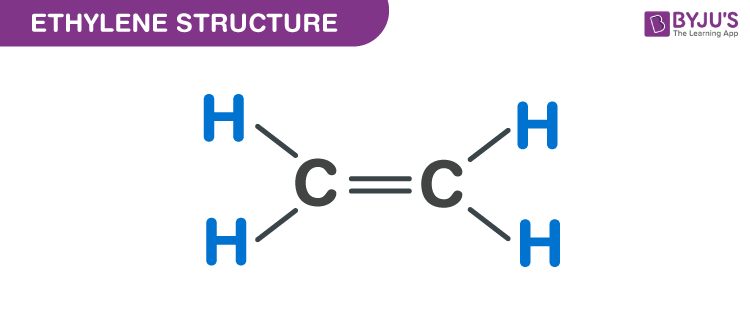
Ethylene C2H4 Structure Molecular Mass Physical and Chemical 
Hydrocarbon Definition Formula Types Video 
General pore features for one step C2H4 production from a C2 
Ethylene Molecule Ethene Hydrocarbon C2h4 Photo Background And 
Hydrocarbons. ppt download 
a What is a homologous series Explain with an example. b State 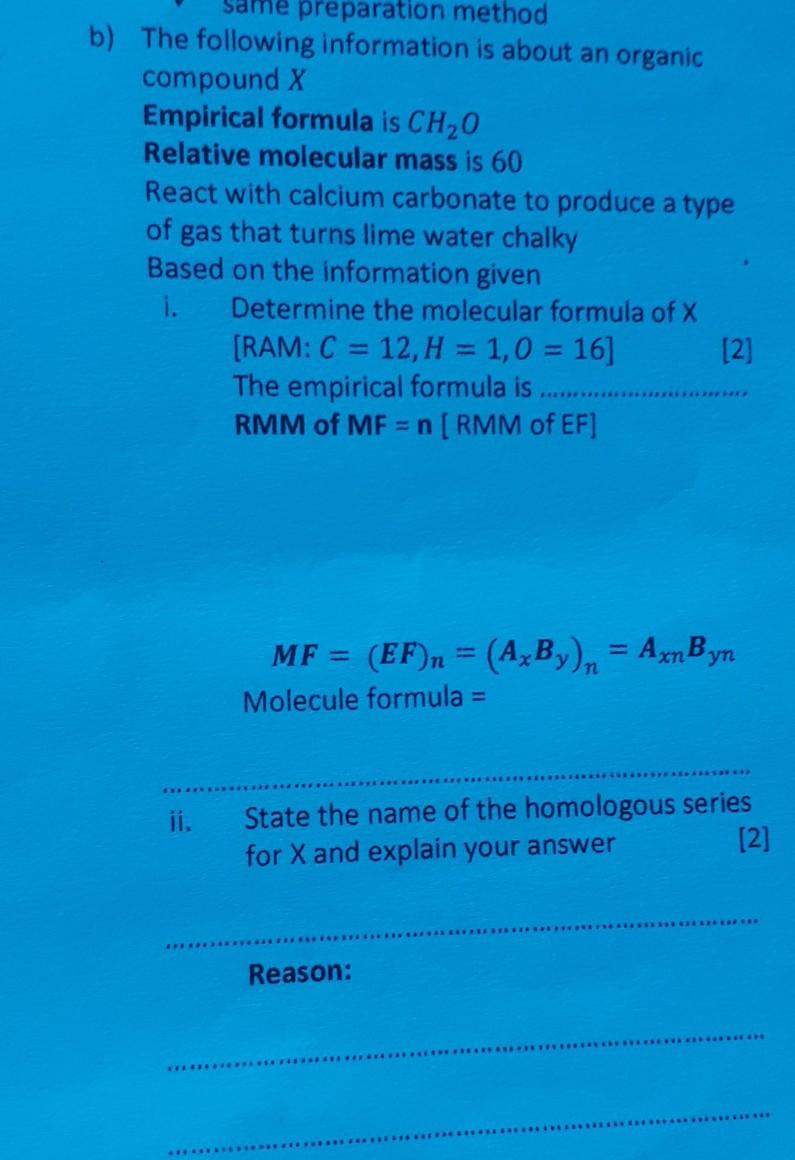
Solved a Table shows some information about three members Chegg 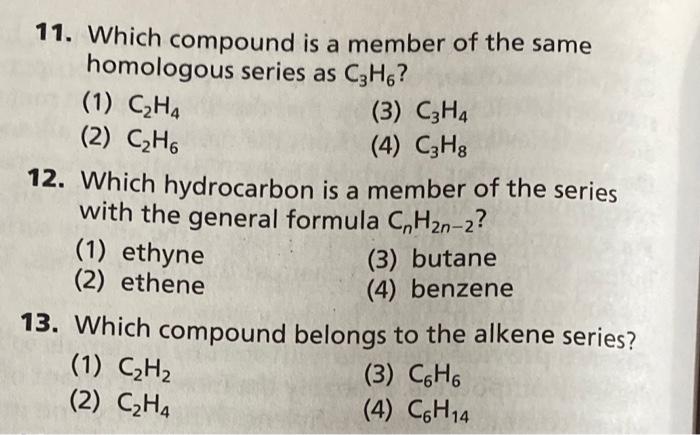
Solved 1. Which element is present in all organic compounds 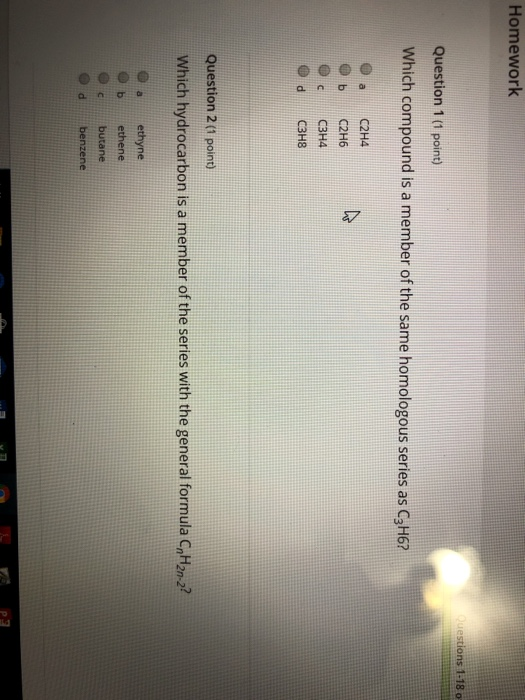
Solved Homework Questions 1 18 0 Question 1 1 point Which 
Ethylene CH2 CH2 CID 6325 PubChem 
Which sequence represents a portion of a homologous series of 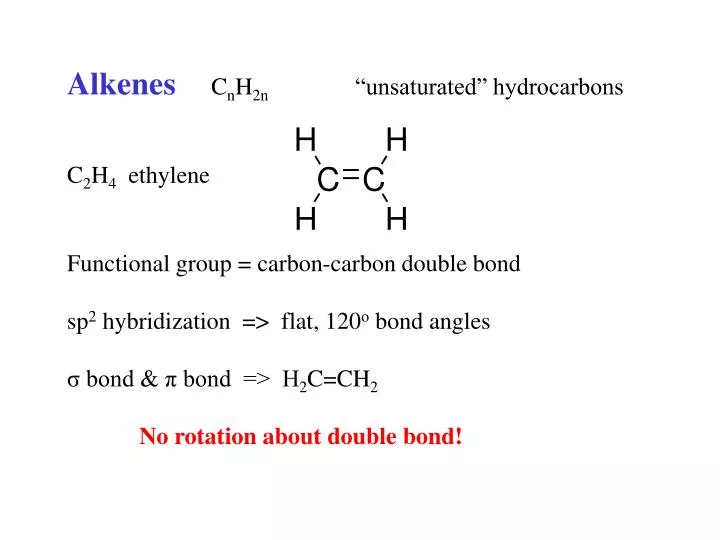
PPT Alkenes C n H 2n unsaturated hydrocarbons C 2 H 4 ethylene 
Alkane Alkene Alkyne Hydrocarbons Structure Properties Video 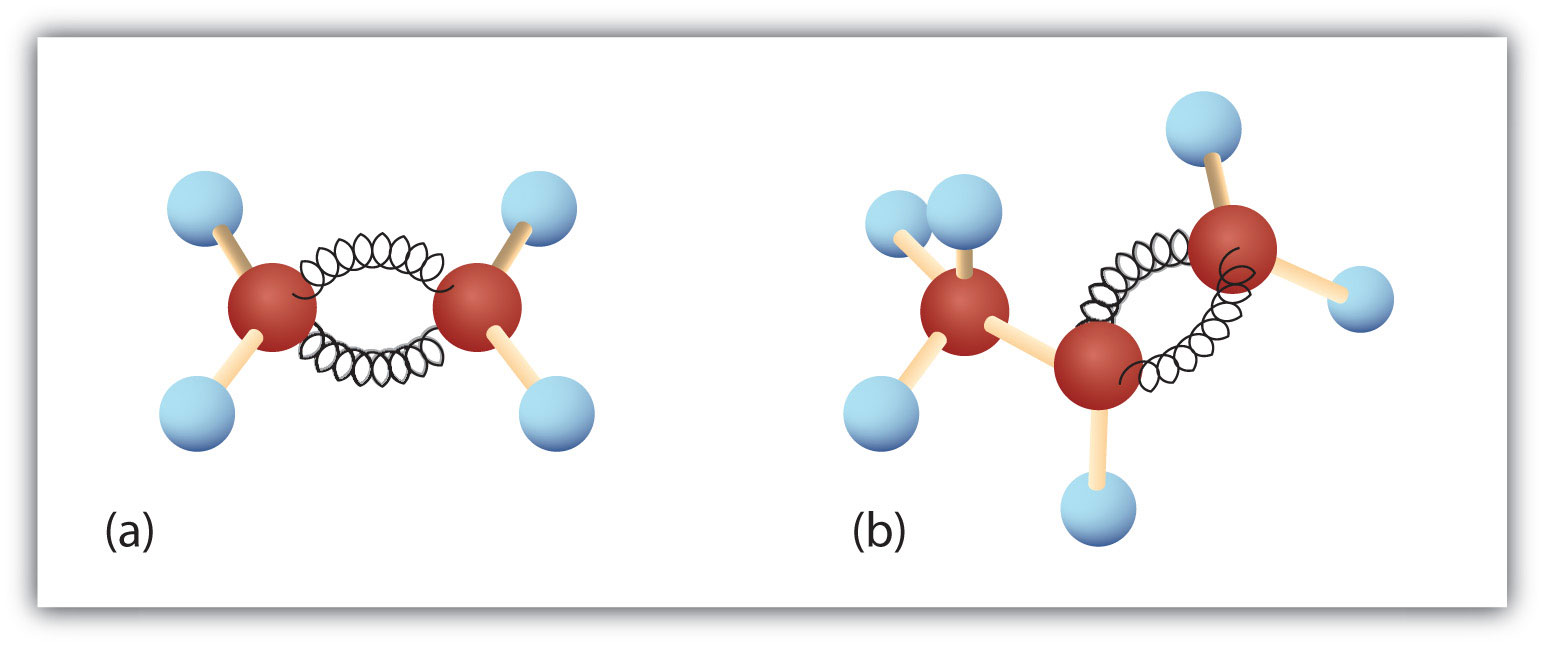
CH105 Chapter 8 Alkenes Alkynes and Aromatic Compounds Chemistry 
PPT Topic Alkenes Alkynes unsaturated hydrocarbons 
PPT Alkenes C n H 2n PowerPoint Presentation free download ID 
Ethylene Wikipedia 
Solved Which Compound Is A Member Of The Same Homologous Series 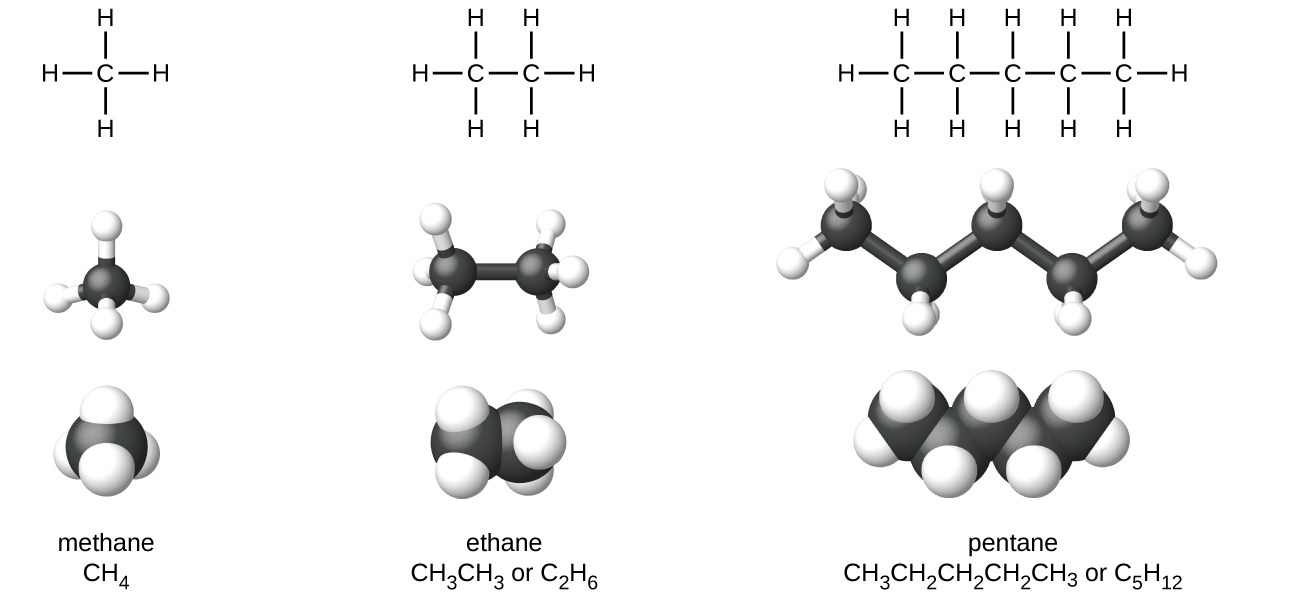
Hydrocarbons Chemistry 
Unsaturated Hydrocarbon Definition Types Examples Lesson 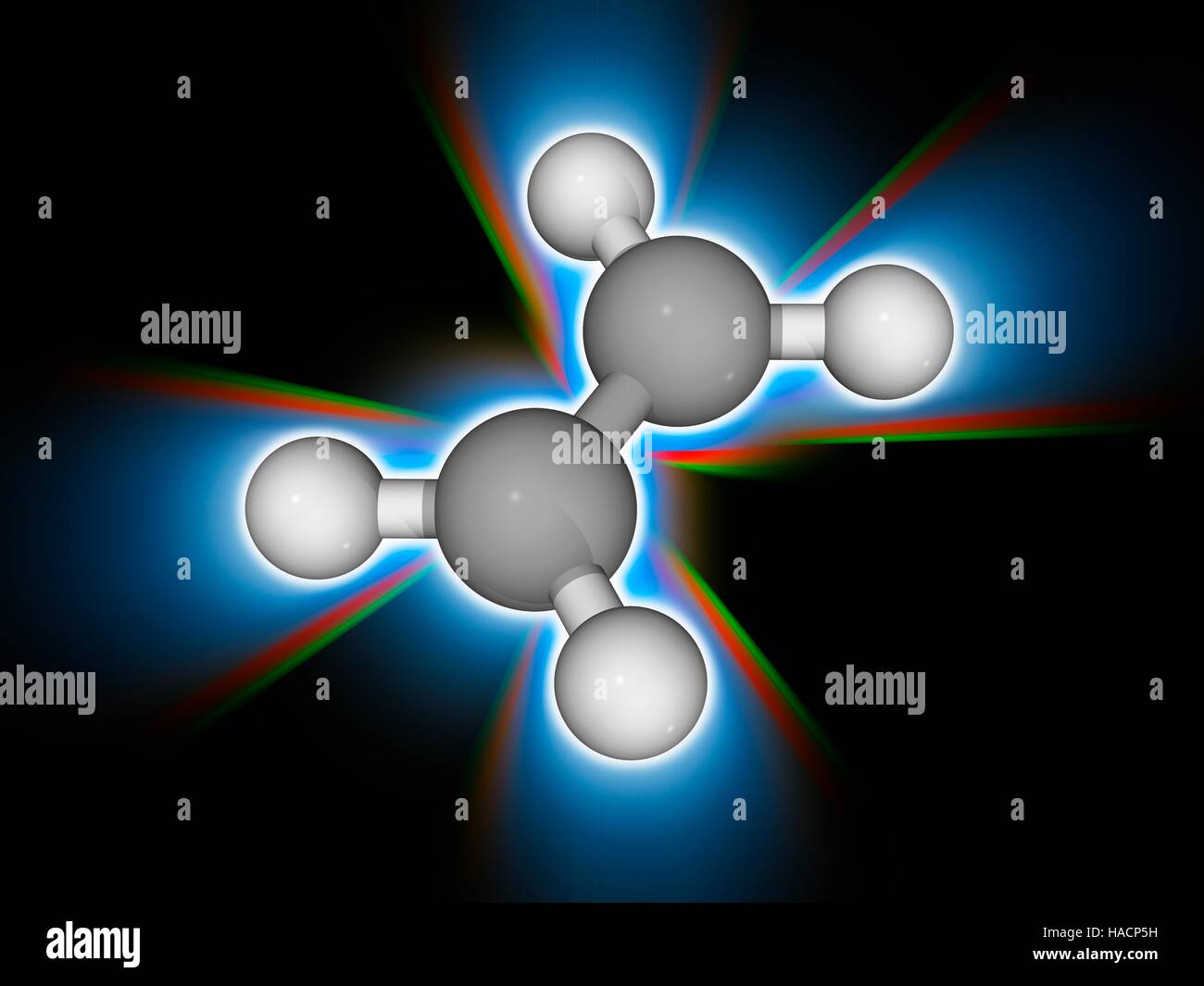
Ethylene. Molecular model of the alkene ethylene C2.H4 also . Ethene, C2H4, is the first member of.jpg)
GCSE Questions and Answers ppt download 
Chemistry of homologous series where first member is ethyne 
Alkene Wikipedia 
What is a homologous series of carbon compounds What are its two 
The formulae of four organic compounds are given below AB C D 
Lesson Explainer Homologous Series Nagwa 
The molecular formulae of four hydrocarbons belonging to a























. Ethene, C2H4, is the first member of.jpg)