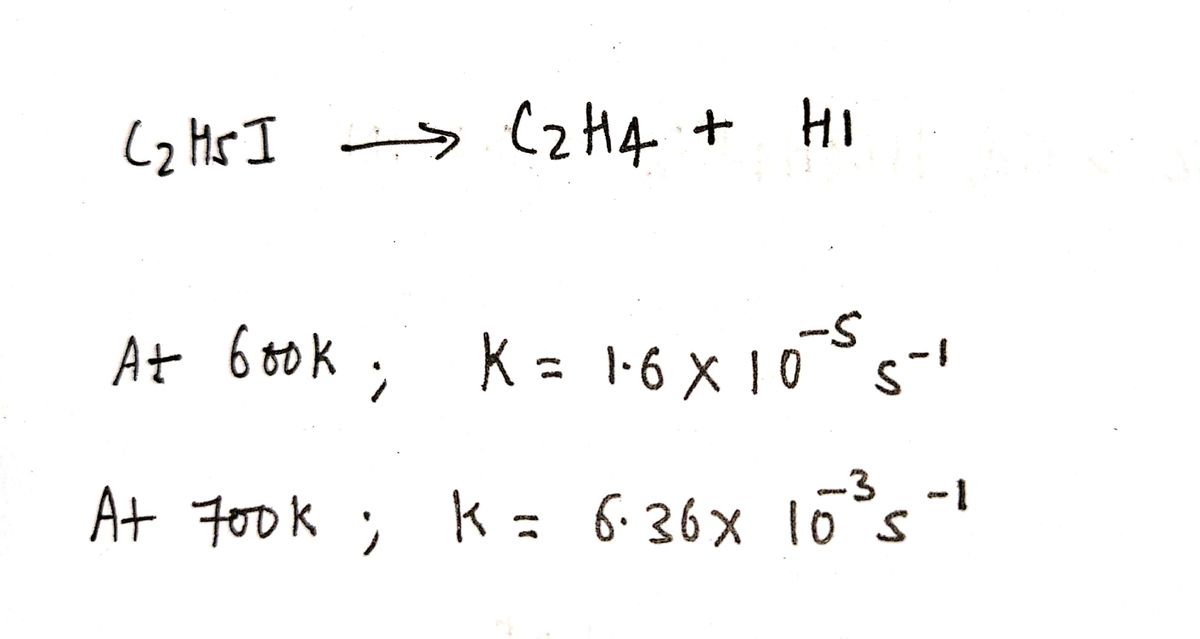What is the formula of pentene Quora
The formula c2h4 hot sale can be classified
Share.
Visit »

Is C2H4 linear or not Quora 
Ethylene CH2 CH2 CID 6325 PubChem 
What is the empirical formula for ethyne C2H2 Quora 
Common Organic Compounds Formula Examples Lesson Study 
What is the formula of ethylene Quora 
Ethylene Wikipedia 
How many covalent bonds are there in a molecule of ethene Quora 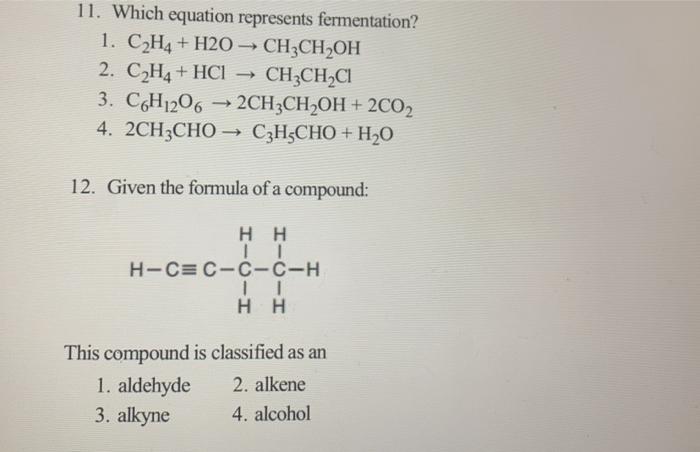
Solved 11. Which equation represents fermentation 1. C2H4 
Name Revsworld 
C2H4 Lewis Structure Ethylene 
Ethylene CH2 CH2 CID 6325 PubChem 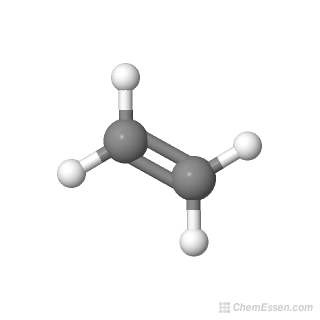
ETHYLENE Formula C2H4 Over 100 million chemical compounds CCDDS 
Alkane Alkene Alkyne Hydrocarbons Structure Properties Video 
Ethylene 9002 88 4 wiki 
Answered HNO3 aq is classified as a strong bartleby 
Solved Which of the following is an empirical formula A Chegg 
What is the formula of pentene Quora 
What is the general formula of alkenes Identify the alkenes from 
Pure 99.95 Industrial Liquid C2h4 Gas Ethylene Gas Price China 
How is the structural formula for C2H4 determined Quora 
ETHYLENE Formula C2H4 Over 100 million chemical compounds CCDDS 
Solved From Table 2.1 choose the correct classification for 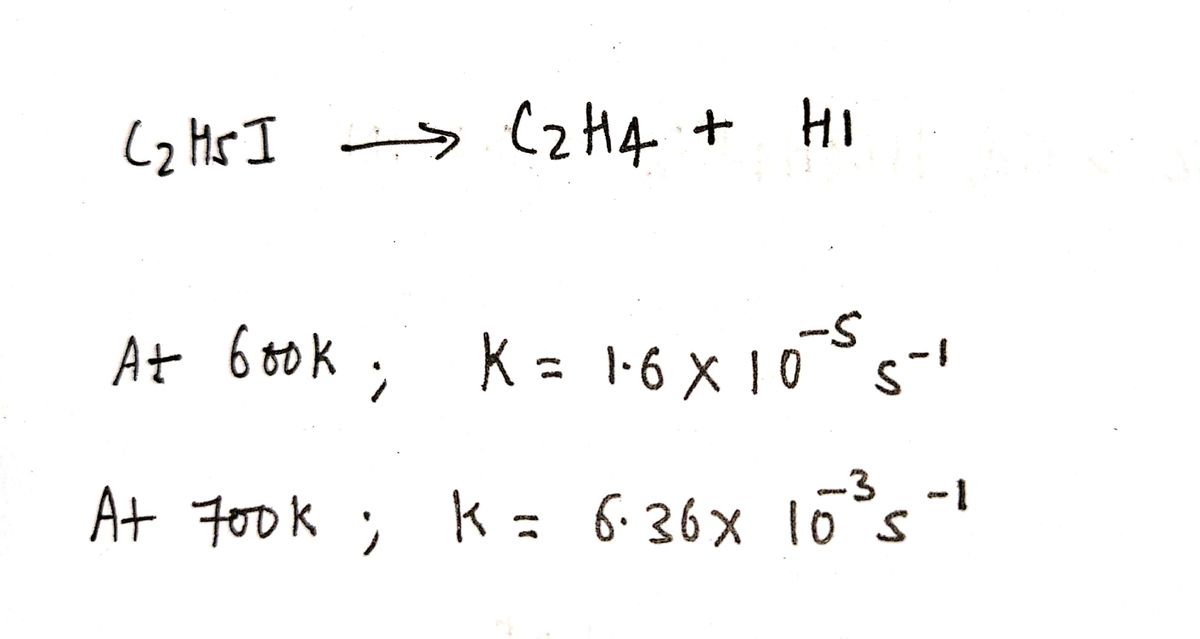
Answered For the reaction C2H5I C2H4 HI bartleby 
The formula C2H4 can be classified as 1 a structural formula 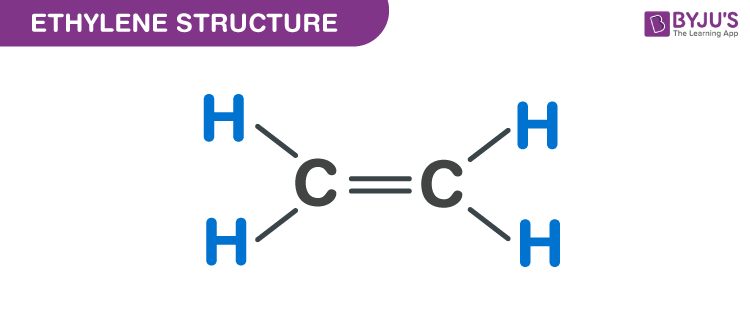
Ethylene C2H4 Structure Molecular Mass Physical and Chemical 
When conducting an aldol reaction with the following ketone there 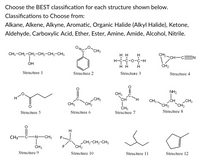
Answered Choose the BEST classification for each bartleby 
The formula C2H4 can be classified as 1 a structural formula 
Ethylene Wikipedia 
Draw the electron dot structure for C2H4. What is its molecular 
How is the structural formula for C2H4 determined Quora 
SOLUTION Chem 120 week 5 lab organic chemistry Studypool 
How is the structural formula for C2H4 determined Quora 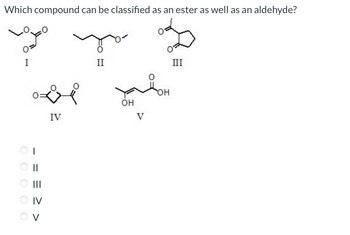
Answered Which compound can be classified as an bartleby 
Determine the empirical formula for the compound represented by each molecular formula a C2H4 b C 
Common Organic Compounds Formula Examples Lesson Study 
Chemical compound Elements Molecules Reactions Britannica 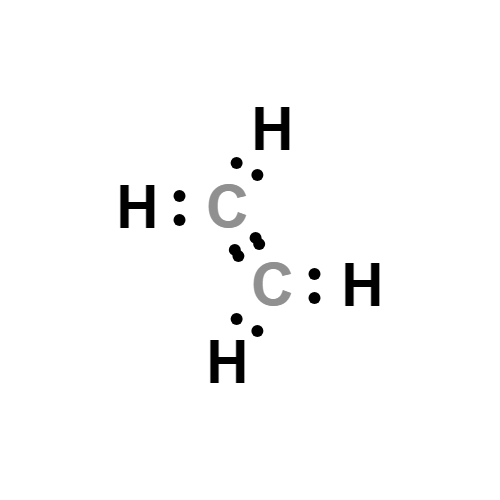
ETHYLENE 74 85 1 
Draw and explain the Lewis structure of C2H4. Homework.Study