Calculate the ethalpy of combustion of ethylene g to form CO 2 gas and H 2 O gas at
Standard enthalpy of formation of ethylene c2h4 hot sale g
Share. Visit »
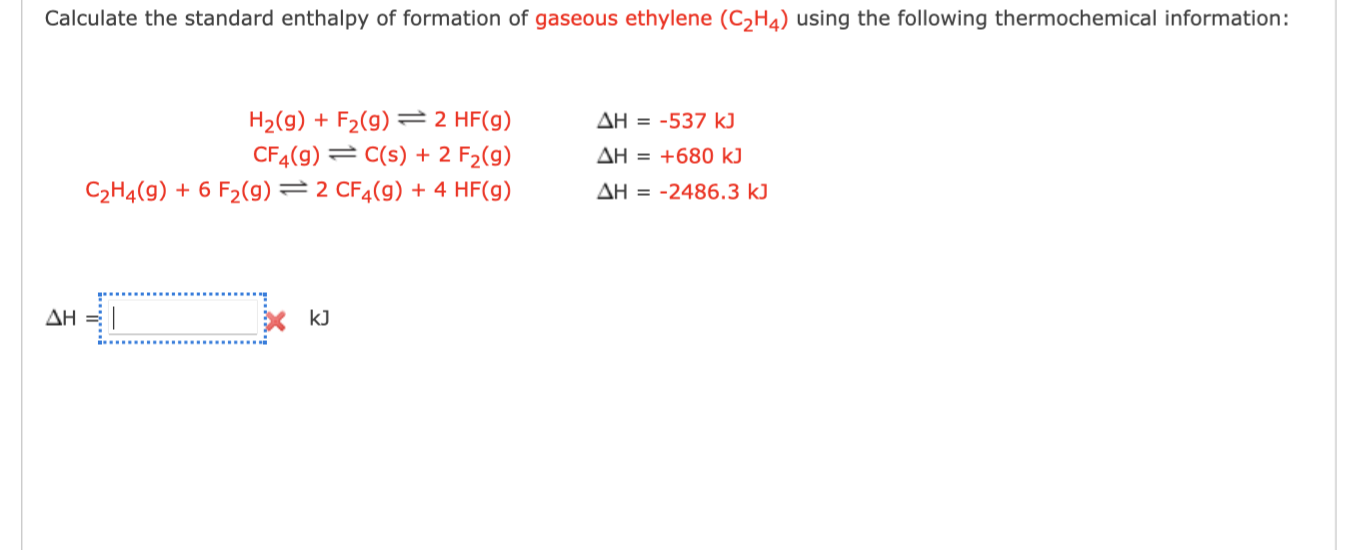
3.Calculate the standard enthalpy of formation of C2H4 g from the
Solved Calculate the standard enthalpy of formation of Chegg
ACS Exam General Chemistry Energetics 19 Calculate the enthalpy of combustion of ethylene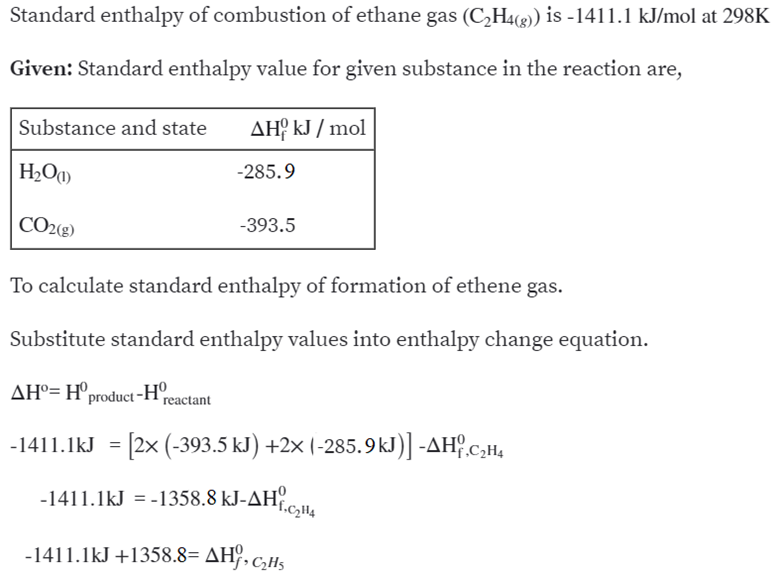
Answered The standard enthalpy of combustion of bartleby
SOLVED Find the standard enthalpy of formation of ethylene C2H4
ASSIGNED READINGS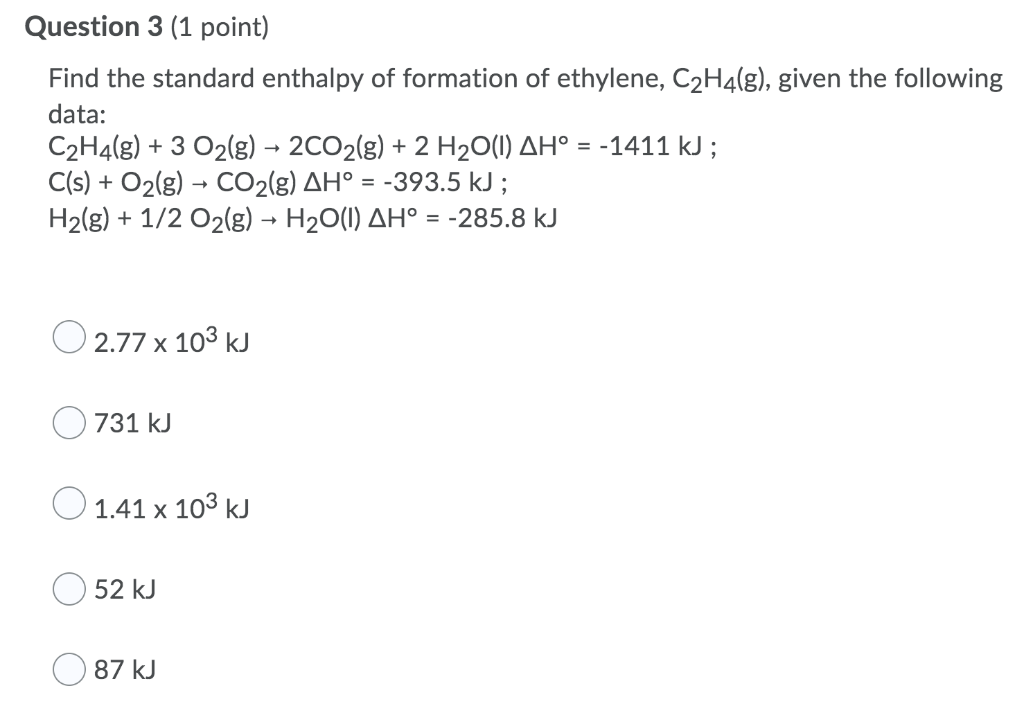
Solved Question 3 1 point Find the standard enthalpy of Chegg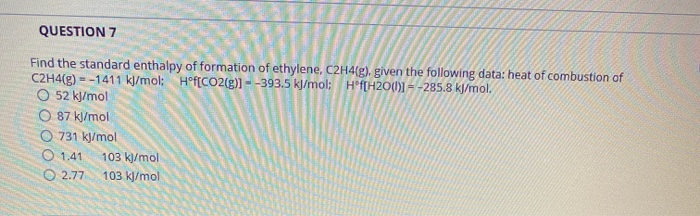
Solved QUESTION 7 Find the standard enthalpy of formation of
In C2H4 formation of C C and C C is 590 kJ mole and 331
Calculate the enthalpy of combustion of ethylene 1 atm pressure
Calculate the heat of reaction 25 C the reaction C2H4 g H2 g
property 27. Calculate the heat of reaction 298K the reaction C2H4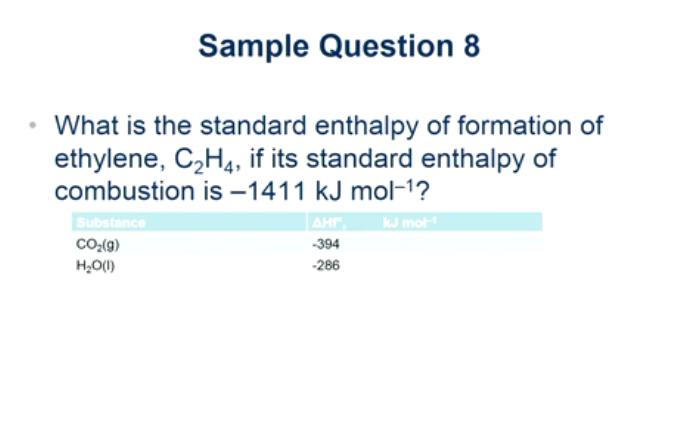
Solved Sample Question 8 What is the standard enthalpy of Chegg
calculate the standard enthalpy of formation of ethene C2H4 from
42. Standard enthalpies of combustion of C2H4 g C2H6 g and H2 g
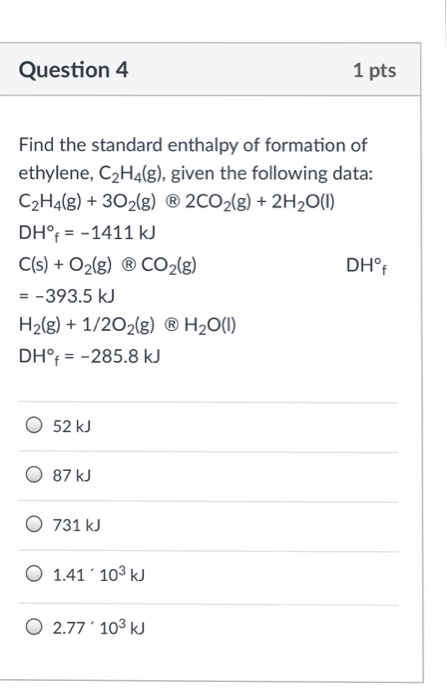
Solved Question 4 1 pts Find the standard enthalpy of Chegg
Calculate enthalpy formation of ethylene from the following data
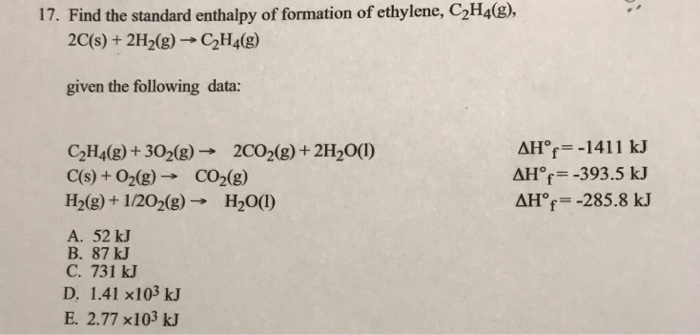
Solved 17. Find the standard enthalpy of formation of Chegg
Calculate the heat of combustion of ethylene to form co and H O
property 27. Calculate the heat of reaction 298K the reaction C2H4
ntif enthalpies of formation for c2h4 g co2 g and h2o l at
Solved the standard enthalpy of formation of ethylene Chegg
Calculate the ethalpy of combustion of ethylene g to form CO 2 gas and H 2 O gas at
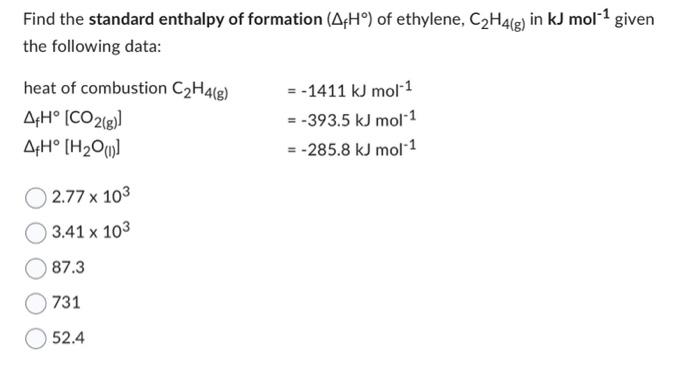
Solved Find the standard enthalpy of formation fH of Chegg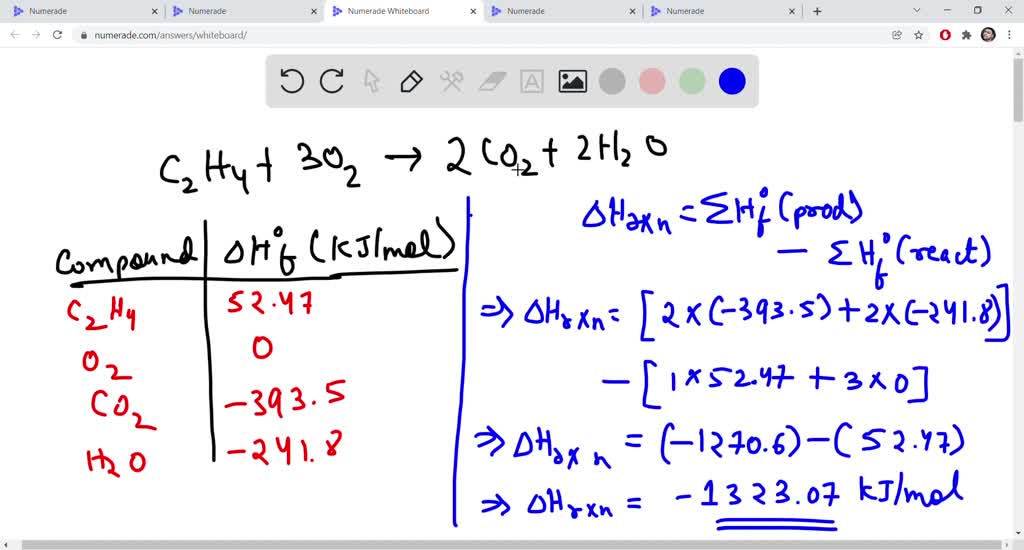
SOLVED The combustion of ethene C2H4 occurs via the reaction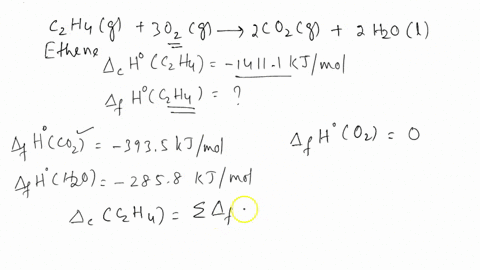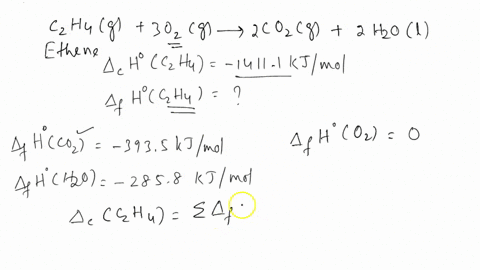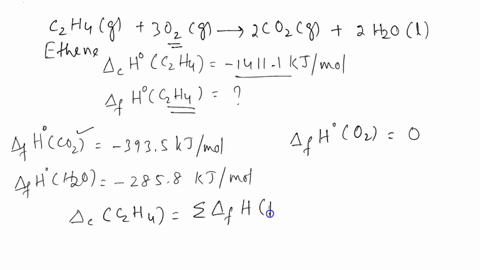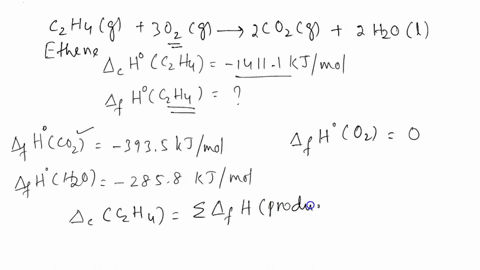
SOLVED The standard enthalpy of combustion of ethene gas C2H4 g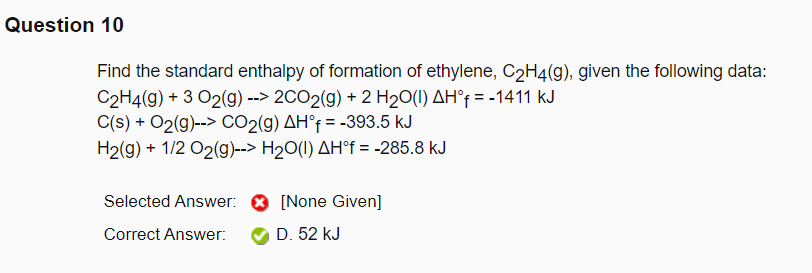
Solved Question 10 Find the standard enthalpy of formation Chegg
Answered 5. Write a balanced thermochemical bartleby
Answered For the combustion reaction of ethylene bartleby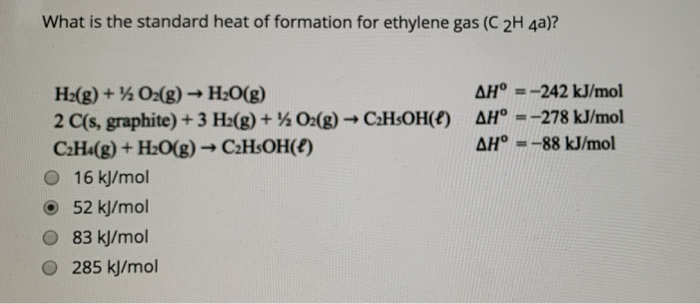
Solved What is the standard heat of formation for ethylene Chegg
Solved QUESTION 3 Find the standard enthalpy of formation of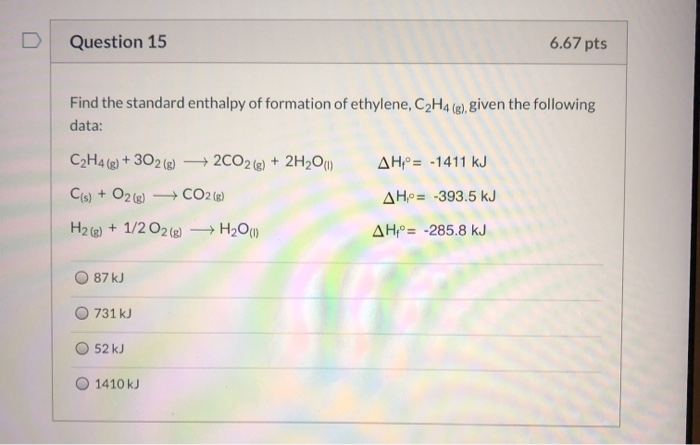
Solved Question 15 6.67 pts Find the standard enthalpy of Chegg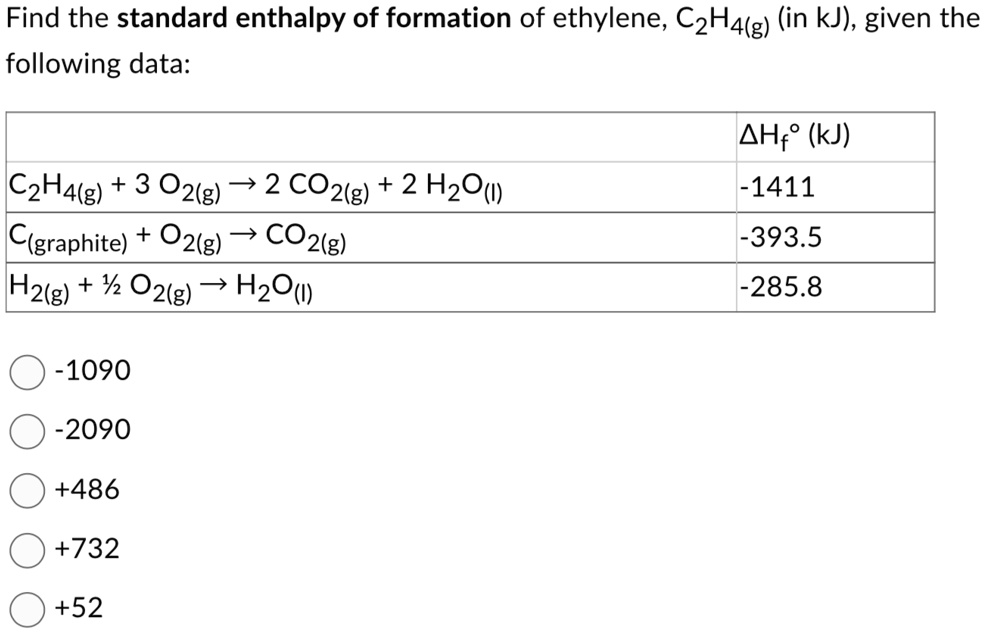
SOLVED Find the standard enthalpy of formation of ethylene C2H4
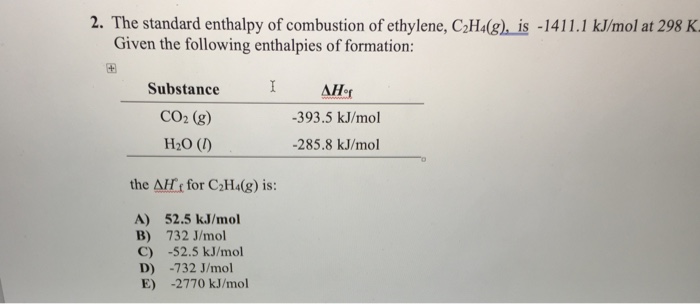
Solved 2. The standard enthalpy of combustion of ethylene Chegg
Calculate the enthalphy of formation for C2H4. DHf CO2 393.5 kJ mol DHf H2O 285.8 kJ mol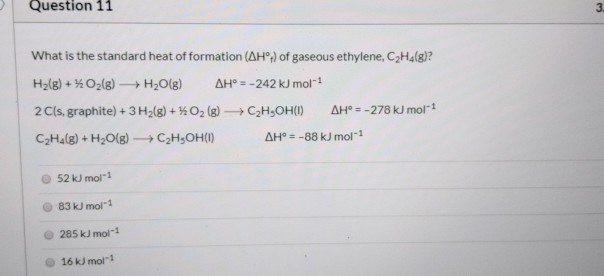
Solved Question 11 3 What is the standard heat of formation
Find the standard enthalpy of formation of ethylene Chegg
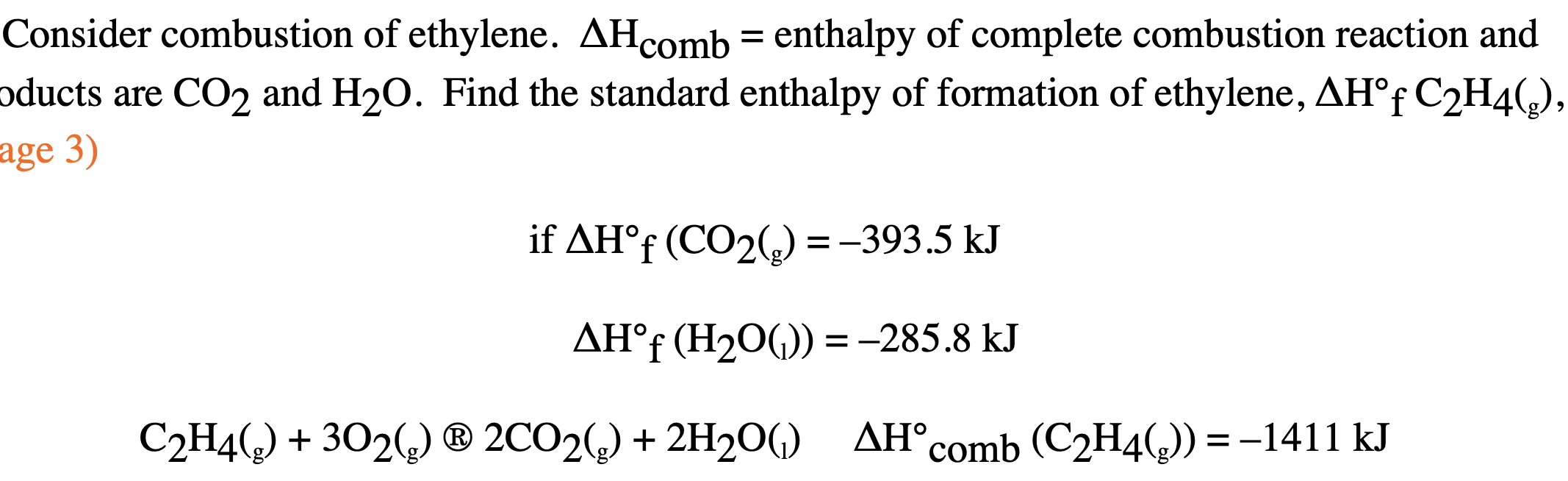
Solved Consider combustion of ethylene. AH comb enthalpy Chegg
Solved 17. Find the standard enthalpy of formation of Chegg