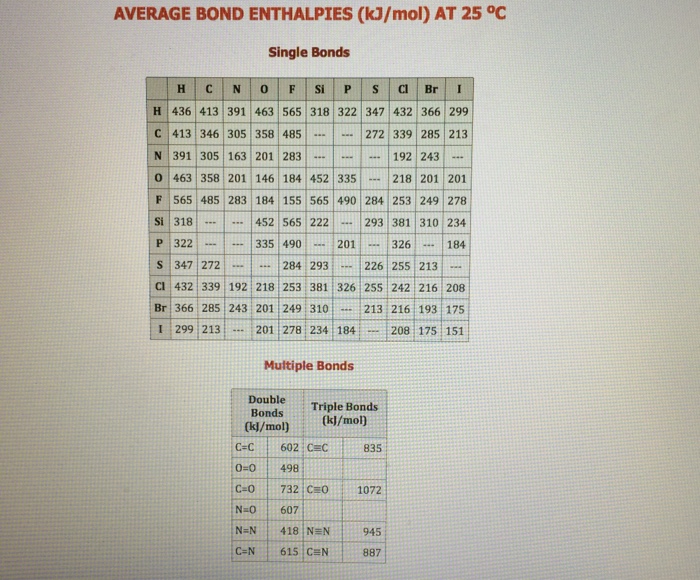
Answered Use the standard molar entropies bartleby
Standard molar hot sale entropy of c2h4
Share. Visit »
Chapter 8 Entropy Free Energy and the Second Law of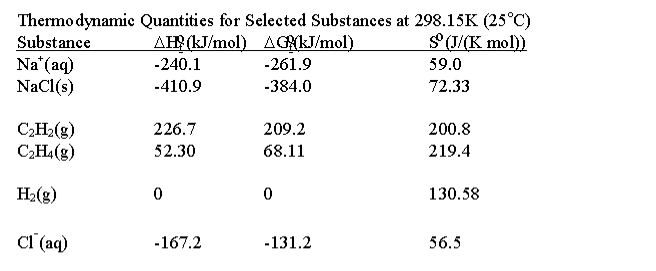
Another Thermo Question Socratic
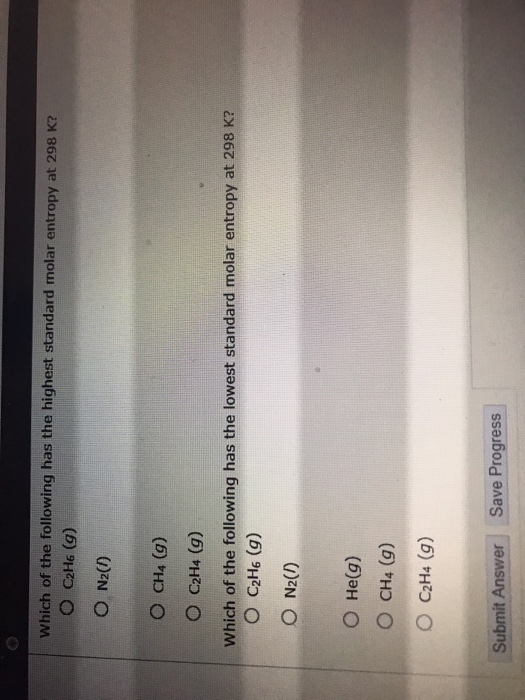
Solved Which of the following has the highest standard molar
Describe qualitatively how standard enthalpy and entropy of
Measuring Entropy and Entropy Changes Introductory Chemistry
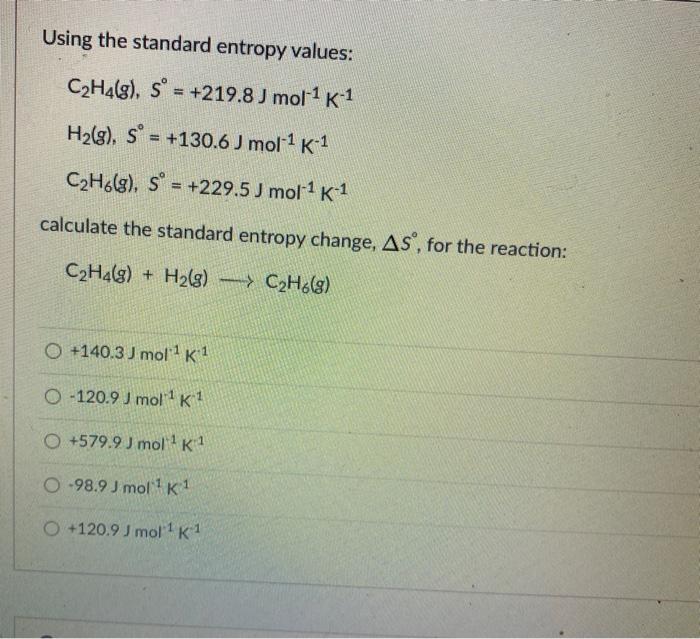
Solved Using the standard entropy values C2H4 g s Chegg
Chapter 17 PDF Gibbs Free Energy Chemical Equilibrium
Use the values of of G f in Appendix B to calculate the stan dar
Solved Calculate the standard entropy change for the following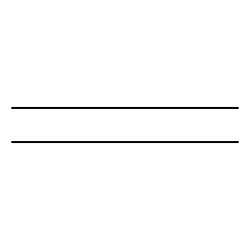
Ethylene CAS 74 85 1 Chemical Physical Properties by Chem o
SOLVED Given the table for standard molar entropy values of each
42. Standard enthalpies of combustion of C2H4 g C2H6 g and H2 g
Solved Standard Entropy of Reaction 1 Chegg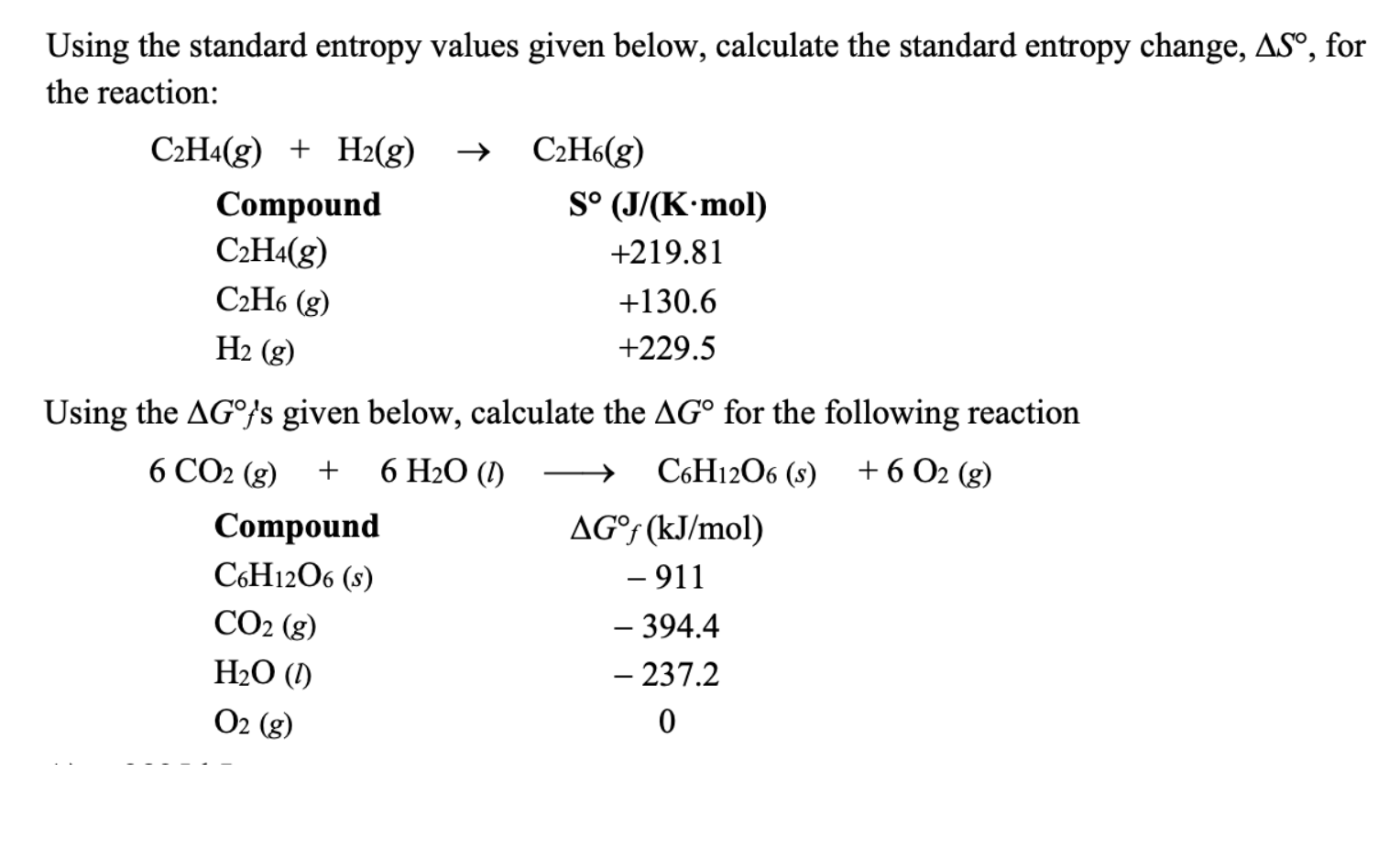
Solved Using the standard entropy values given below Chegg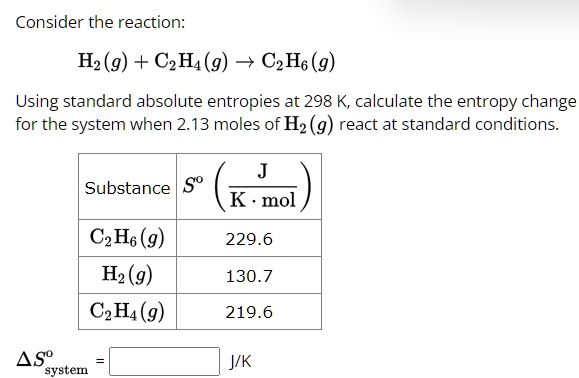
SOLVED Consider the reaction H 2 g C 2 H 4 g C 2 H 6 g
Solved Arrange these compounds in order of increasing Chegg
Calculate the S rxn of the following reaction at 215 C and
Ethylene Thermophysical Properties
Solved Calculate the standard entropy change for the following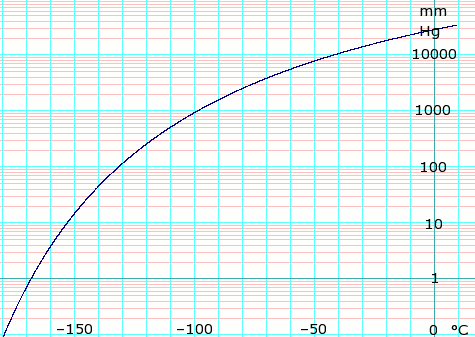
Ethylene data page Wikipedia
Calculate the enthalphy of formation for C2H4. DHf CO2 393.5 kJ mol DHf H2O 285.8 kJ mol
Second law of Thermodyna mics 2. If an irreversible process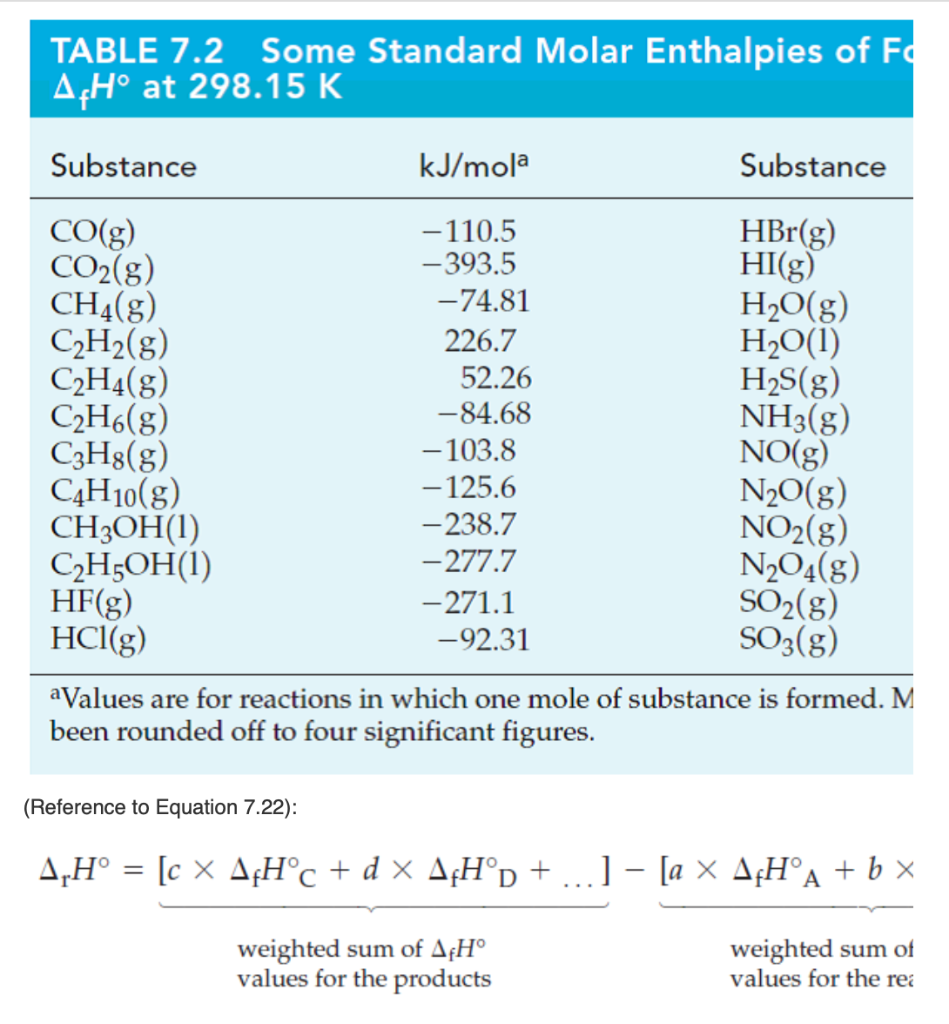
Solved TABLE 7.2 Some Standard Molar Enthalpies of Fa AcH Chegg
OneClass Could you please explain how to determine ranking the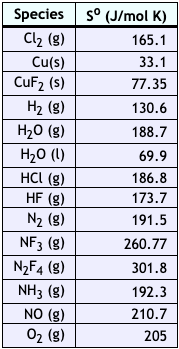
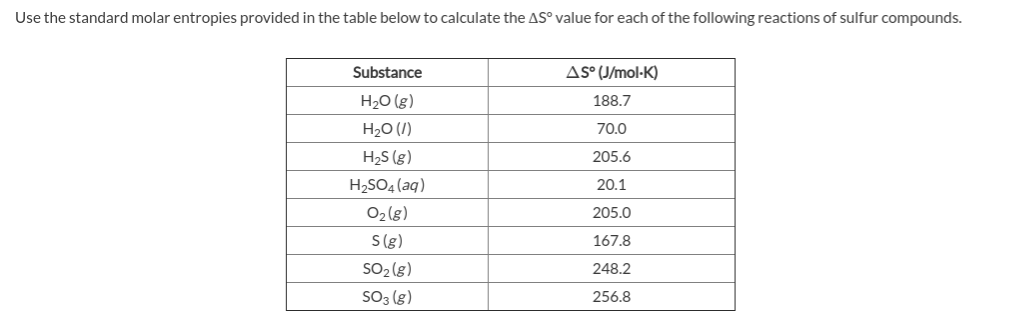
Answered Use the table of standard entropies bartleby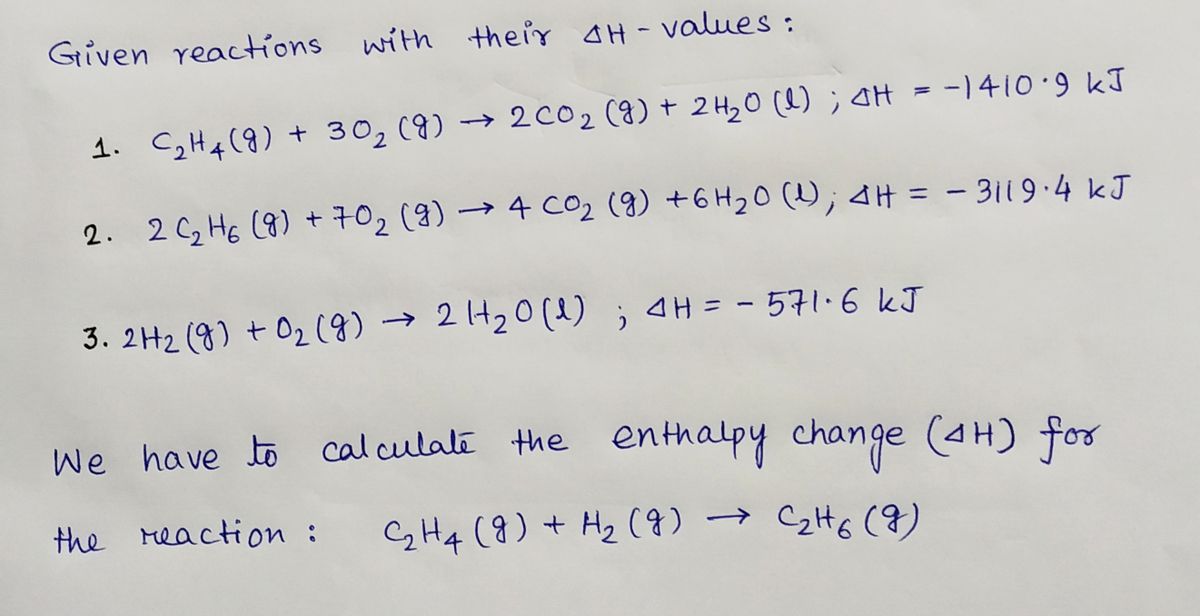
Answered Calculate the enthalpy change for the bartleby
Answered 8 a Calculate AS en for the following bartleby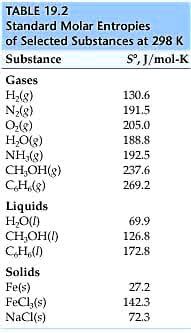
SOLVED TABLE 19.2 Standard Molar Entropies of Selected Substances
Rank the following compounds by standard molar entropy highest to lowest N2g H2g C2H4g
PPT Chapter 16 Thermodynamics Entropy Free Energy and
Standard Entropy Change for a Reaction
Calculate the ethalpy of combustion of ethylene g to form CO 2 gas and H 2 O gas at
Calculate the enthalpy of combustion of ethylene 1 atm pressure
ntif enthalpies of formation for c2h4 g co2 g and h2o l at
Understanding Hess Law C2H4 H2O C2H5OH
OneClass The standard enthalpy of combustion of solid phenol
3 Entropy Notes PDF Chemical Reactions Enthalpy
Solved Which substance has the highest standard molar Chegg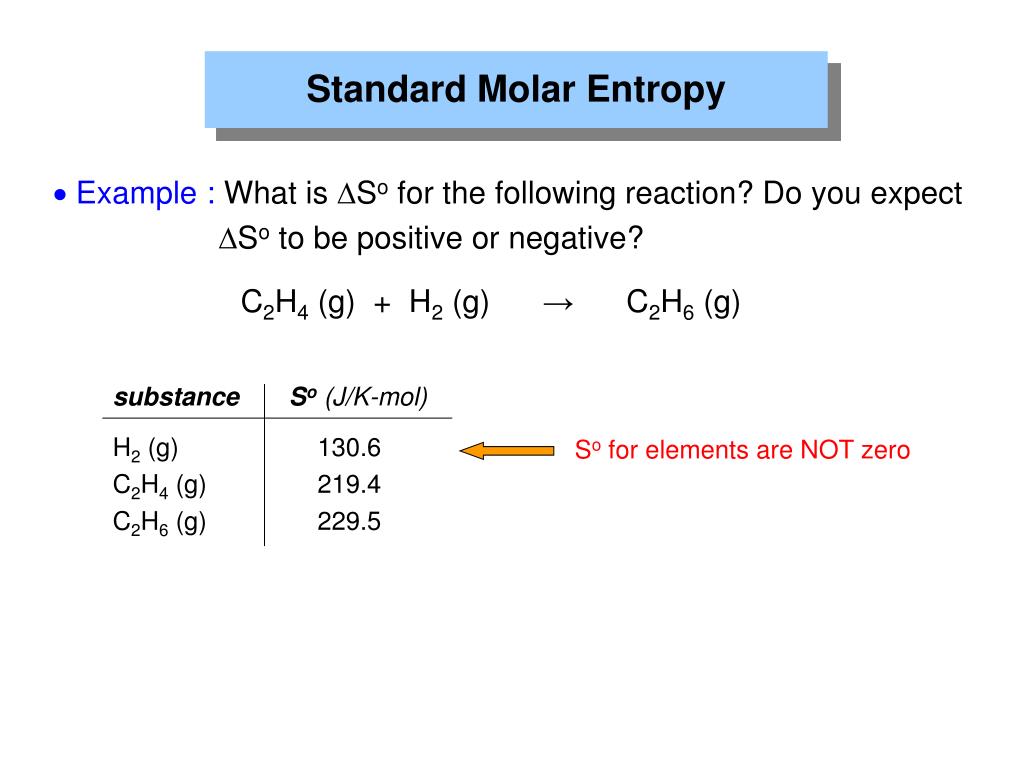
PPT Chemistry 101 Chap. 19 PowerPoint Presentation free