AP CHEMISTRY 2011 SCORING GUIDELINES
The combustion of hot sale c2h4 ap chemistry frq
Share. Visit »
Heat of combustion of CH4 C2H4 C2H6 are 890 1411 1550 kJ mol
How to Solve AP Chemistry Stoichiometry Problems
The Ultimate Study Guide to AP Chemistry Albert.io
Answered For the combustion reaction of ethylene bartleby
Calculate the ethalpy of combustion of ethylene g to form CO 2 gas and H 2 O gas at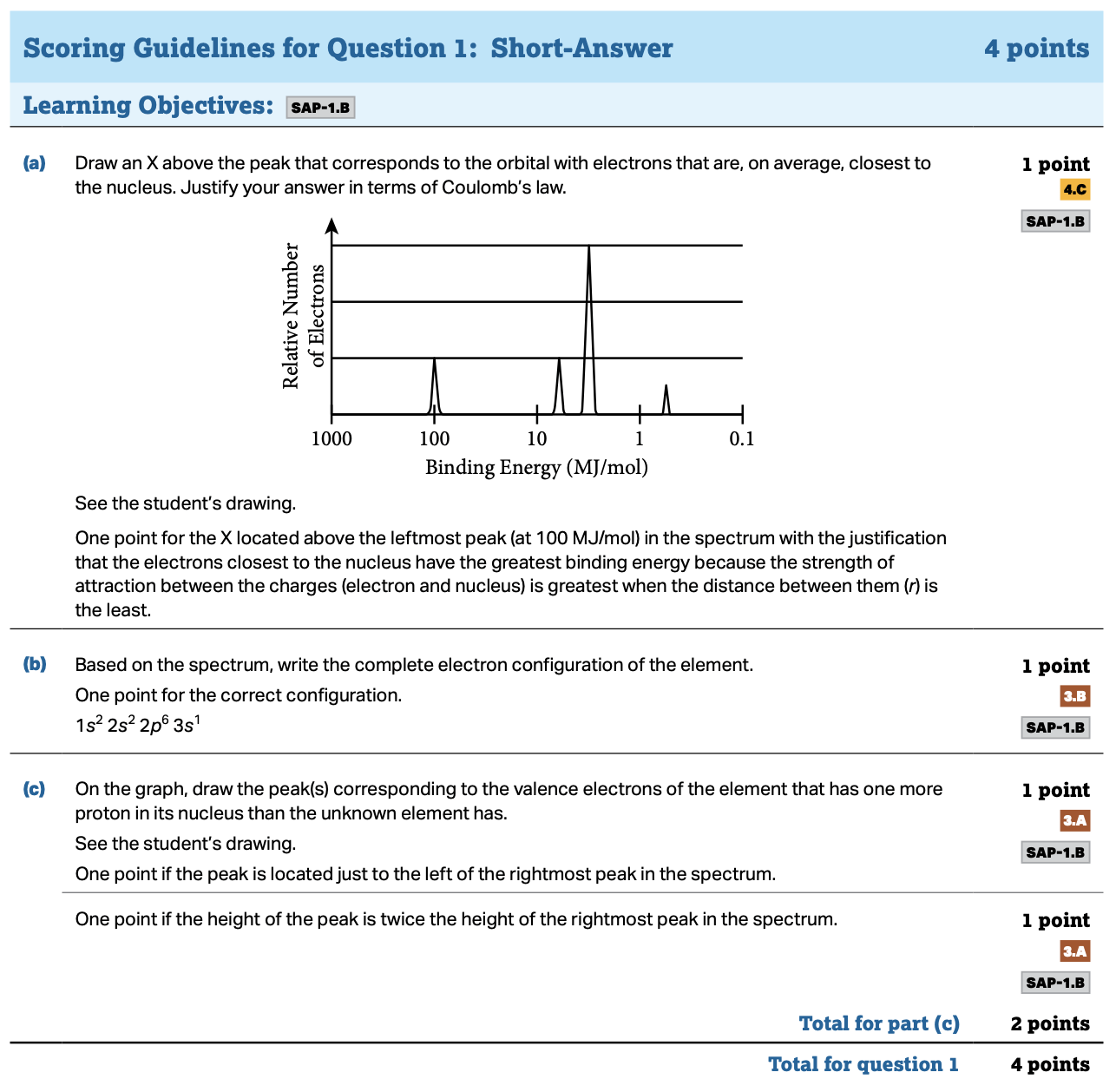
The Best AP Chemistry Review Guide for 2024 Albert Resources
PRACTICE TEST THERMOCHEMISTRY AP CHEMISTRY
AP Chemistry 2012 FRQ 2
Template Unit 6 FRQ Unit 6 FRQ 1 The combustion of C2H4 g is
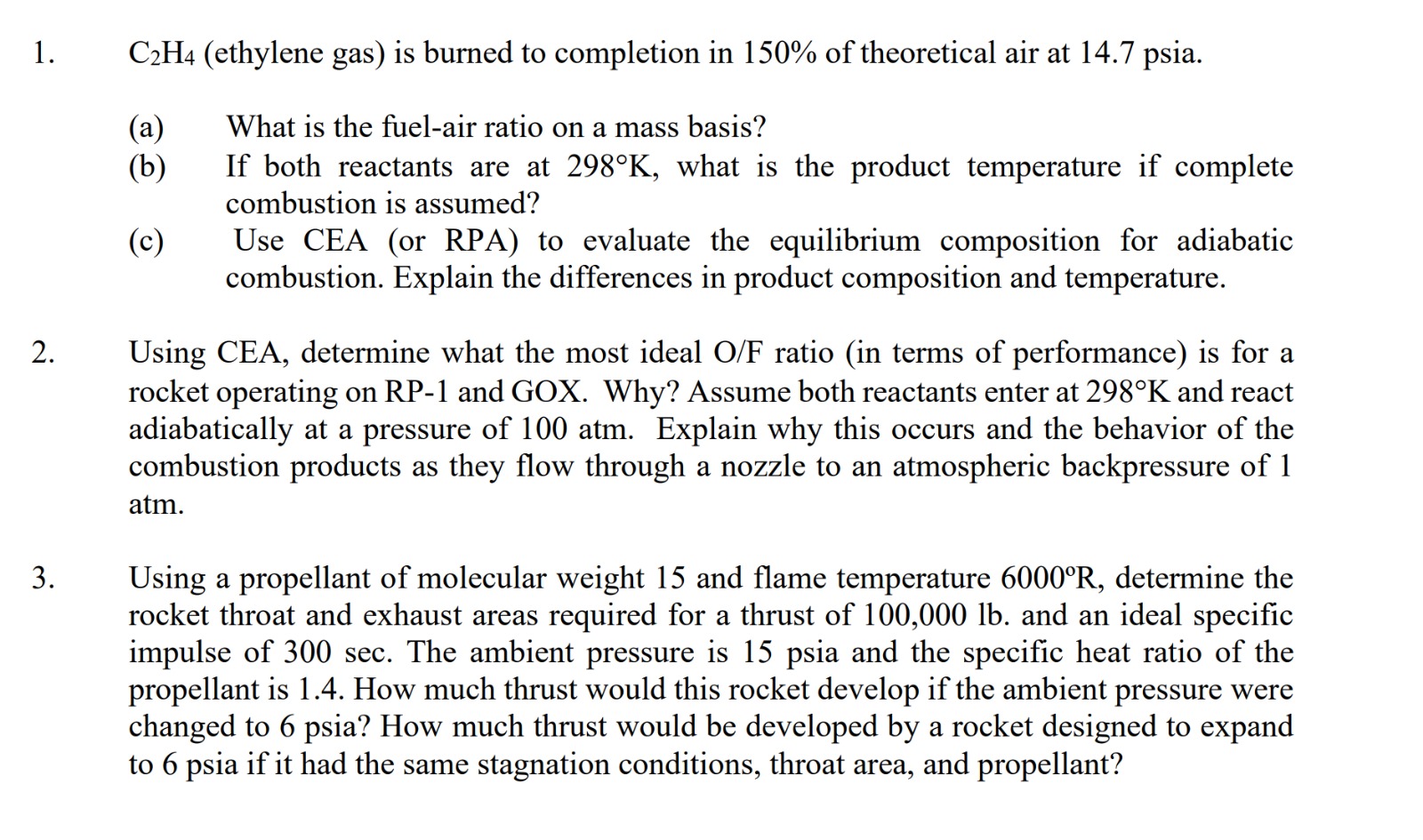
Solved C2H4 ethylene gas is burned to completion in 150 Chegg
Template Unit 6 FRQ Unit 6 FRQ 1 The combustion of C2H4 g is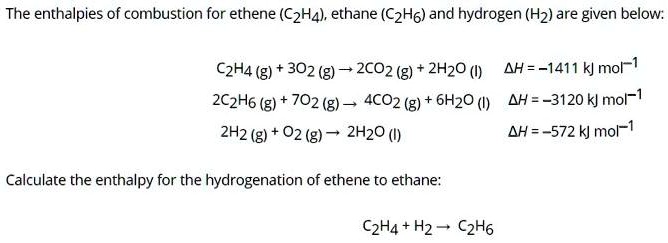
SOLVED The enthalpies of combustion for ethene C2H4 ethane
AP Chemistry Free Response Version A Stoichiometry Please
SLO AP CHEMISTRY Flashcards Quizlet
AP Chemistry Scoring Guidelines from the 2019 Exam Administration
AP Chemistry Chapter 17 Sample Exercises PPT
Ap chemistry master cheatsheet PDF
Use bond energies to confirm that the complete combustion of
2006 AP Chemistry FRQ number 2
Template Unit 6 FRQ Unit 6 FRQ 1 The combustion of C2H4 g is
Answered What is the enthalpy change of the bartleby
Be sure to answer all parts. Calculate the heats of combustion for
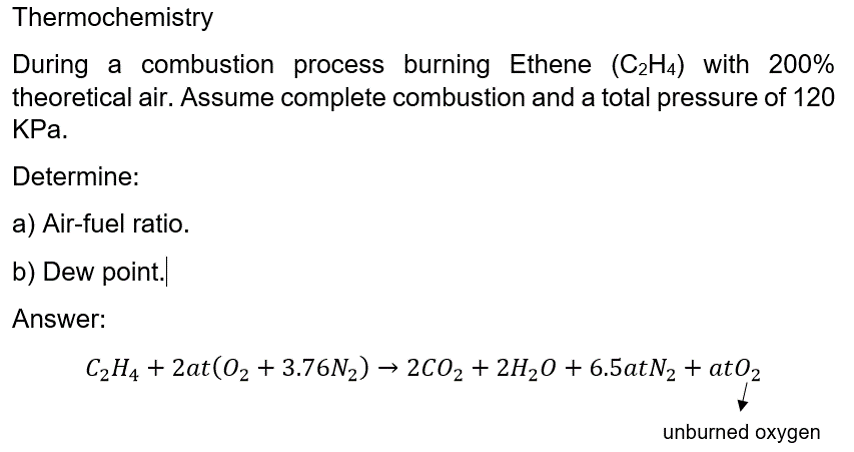
Solved Thermochemistry During a combustion process burning Chegg
AP Chemistry Notes 1.2 Combustion Analysis
42. Standard enthalpies of combustion of C2H4 g C2H6 g and H2 g
2013 AP Chemistry FRQ number 5
Enthalpy vs. Entropy AP Chemistry Crash Course Review
C H OH C H H O 45.5 kJ mol 126 J K mol ethanol
Ethylene C2H4 is burned with 20 excess air in an adiabatic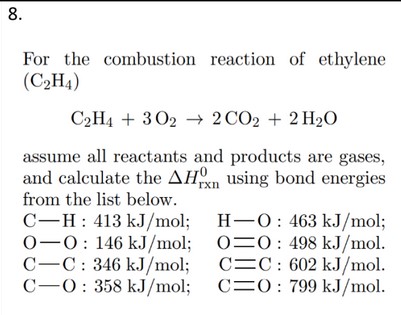
SOLVED 8. For the combustion reaction of ethylene C2H4 C2H4 3
AP Chemistry Sample Student Responses and Scoring Commentary
Untitled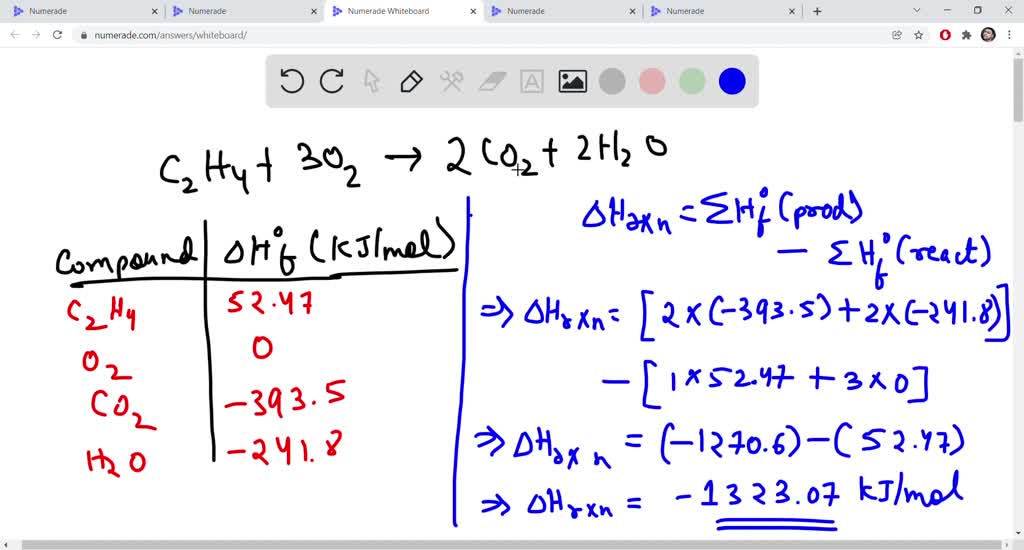
SOLVED The combustion of ethene C2H4 occurs via the reaction
2007 AP Chemistry FRQ number 1
The thermochemical equation for the combustion of ethylene gas C 2 H 4 is C 2 H 4 g
Ap10 Chemistry Scoring Guidelines PDF Chemical Polarity
Ap10 Chemistry Scoring Guidelines PDF Chemical Polarity
Answered The combustion of C2H4 g is bartleby
C H OH C H H O 45.5 kJ mol 126 J K mol ethanol