Structural formula C4H8 having the same molecular formula
The compounds c2h4 and hot sale c4h8 have the same -
Share. Visit »
Homologous series Carbon and its compounds Chemistry Class 10 Khan Academy
Determining the Formula for the Next Compound in a Homologous Series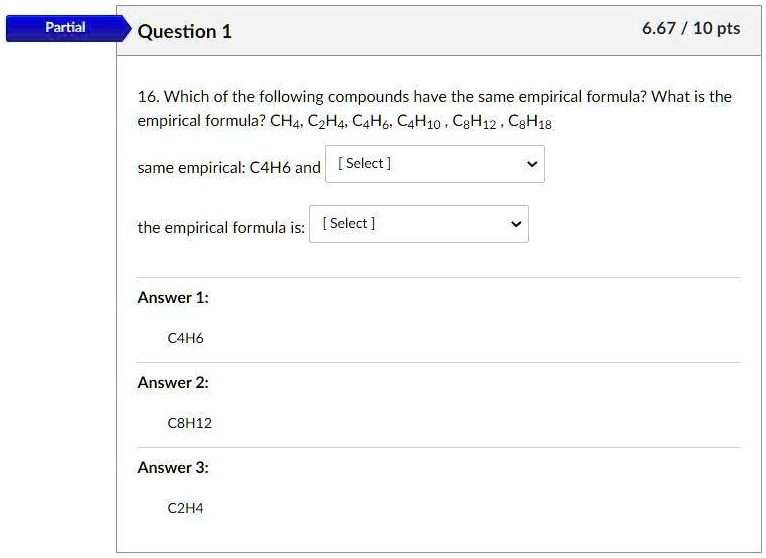
SOLVED 16. Which of the following compounds have the same
Two carbon compounds X and Y have the molecular formula C3H6 and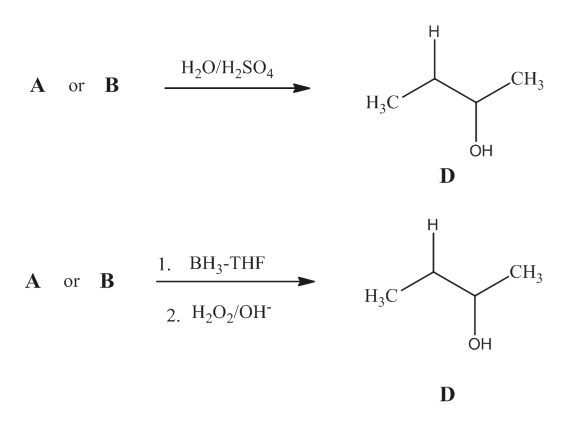
Answered Compounds A B and C have the same bartleby
PPT Alkenes C n H 2n PowerPoint Presentation free download ID
What is the maximum number of open chain isomers that an alkene
Two carbon compounds X and Y have the molecular formula C4H8 and C5H12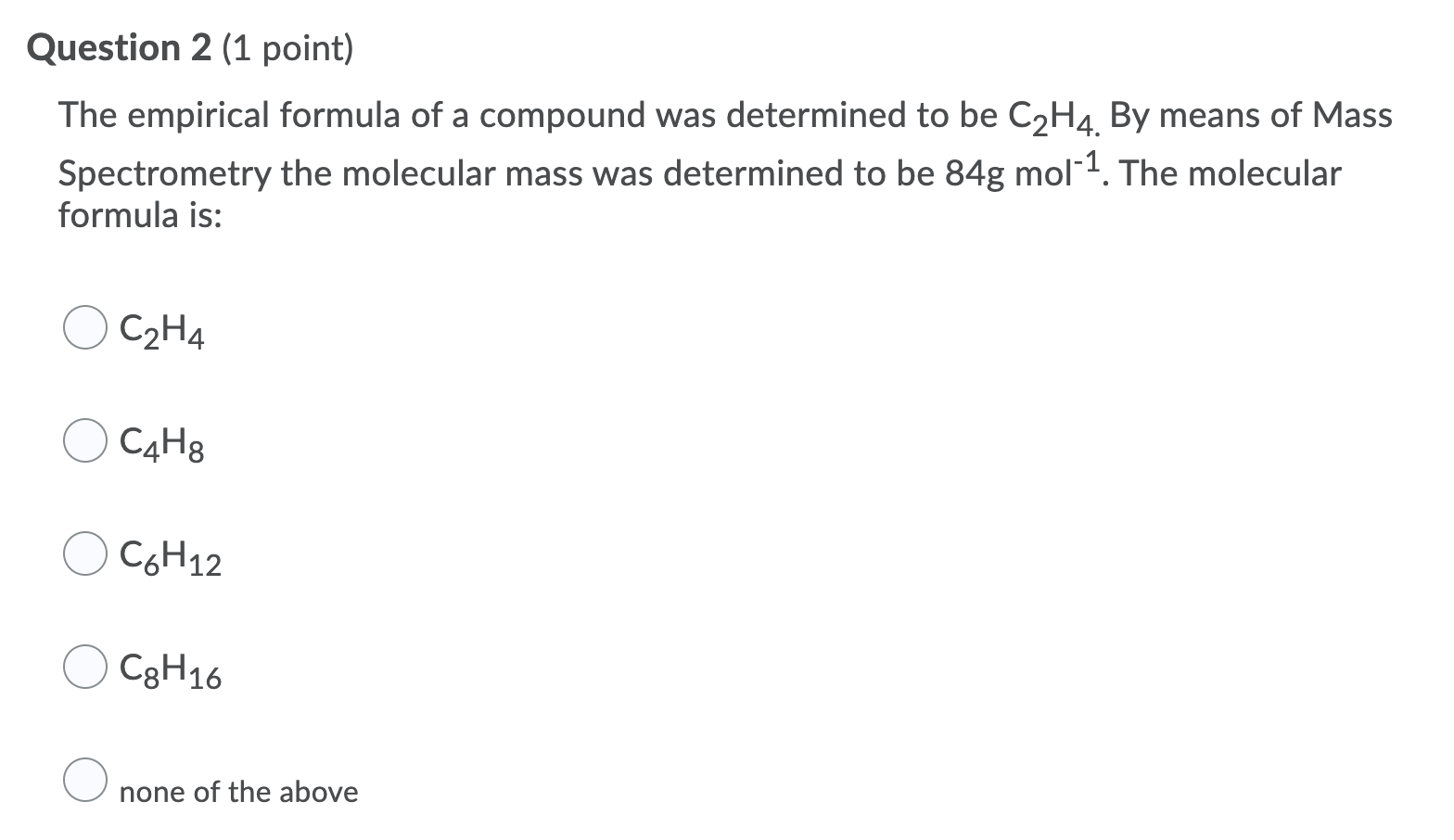
Solved Question 2 1 point The empirical formula of a Chegg
Chemistry SM 1131 Week 8 Lesson 1 ppt download
An alkene with molecular formula C4H8 reacts with bromine to form
Two important industrial chemicals ethene C2H4 and propene
Two important industrial chemicals ethene C2H4 and propene
CHAPTER 4 CARBON AND ITS COMPOUNDS ppt download
classify and tabulate the following compounds into alkanes
Write molecular formula of 2nd 3rd member of homologous series whose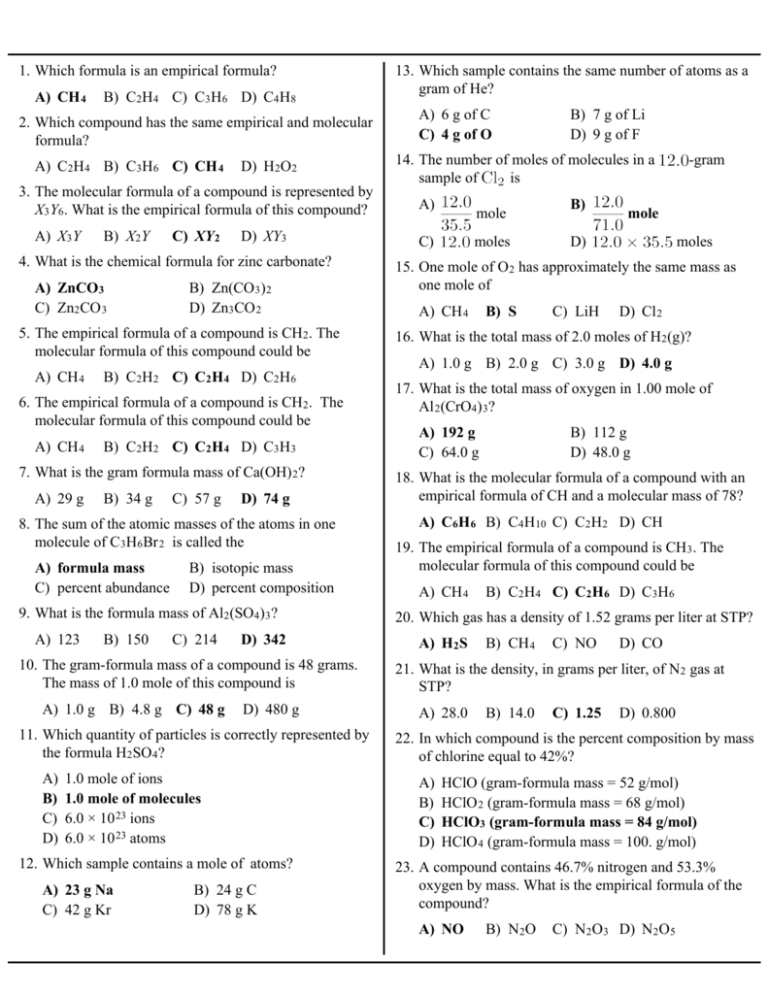
A CH B C2H4 C C3H6 D C4H8 1. Which formula is an empirical
The compounds C2H4 C3H6 C4H8 C5H10 are in homologous series
Answered 2.The only empirical formula is Group bartleby
Define homologous series
Selective synthesis of butane from carbon monoxide using cascade
OR Two carbon compounds X and Y have the molecular formula C H and
Organic Chemistry the study of carbon and most carbon compounds
Structural formula C4H8 having the same molecular formula
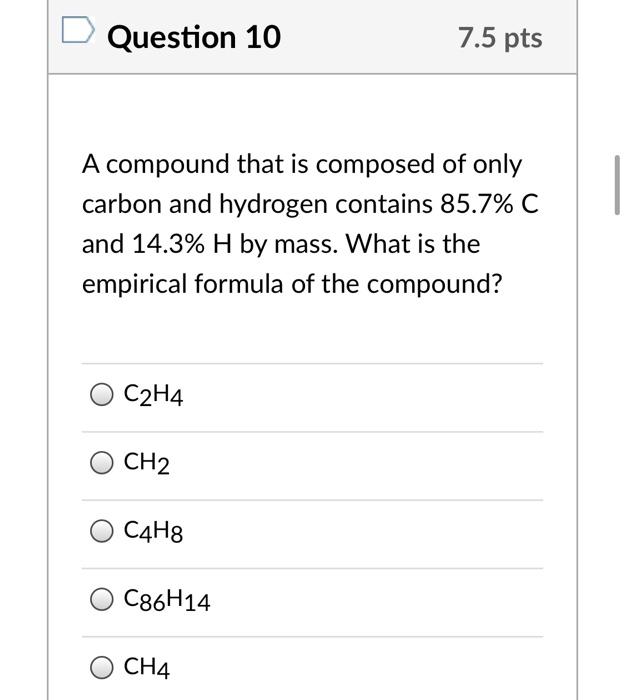
Solved Question 10 7.5 pts A compound that is composed of Chegg
CARBON AND ITS COMPOUNDS. ALKANES Alkanes are hydrocarbons with
Solved This Chemistry 30 Organic chemistry unit. Confused and
4.6 Empirical Formulas Study notes Chemistry Docsity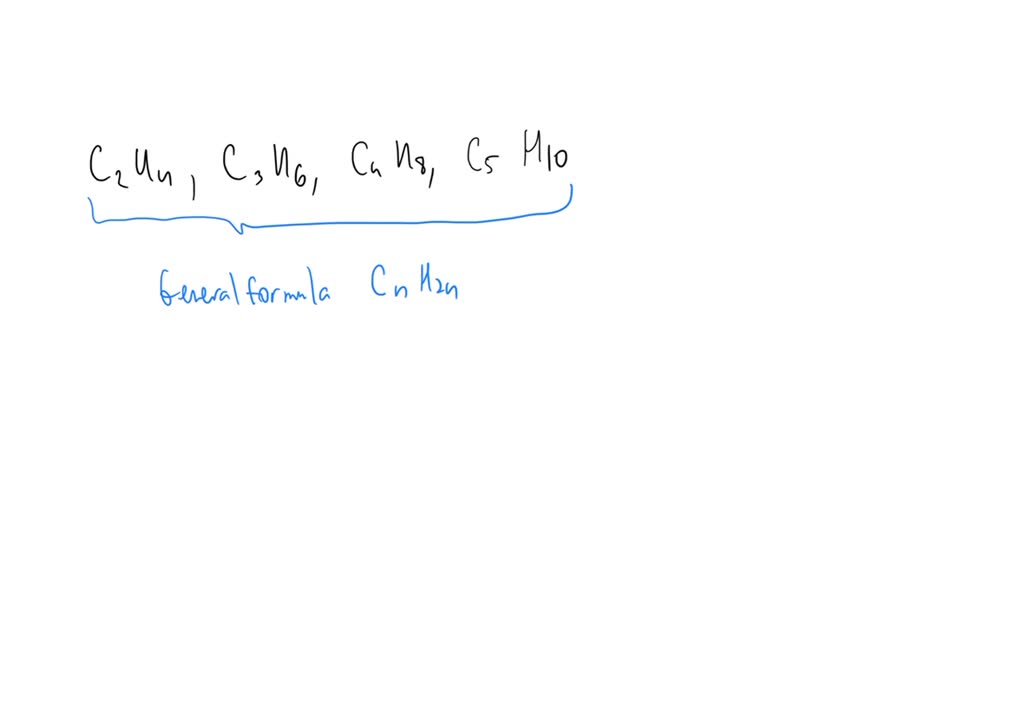
SOLVED The compounds C2H4 C3H6 C4H8 C5H10 are in homologous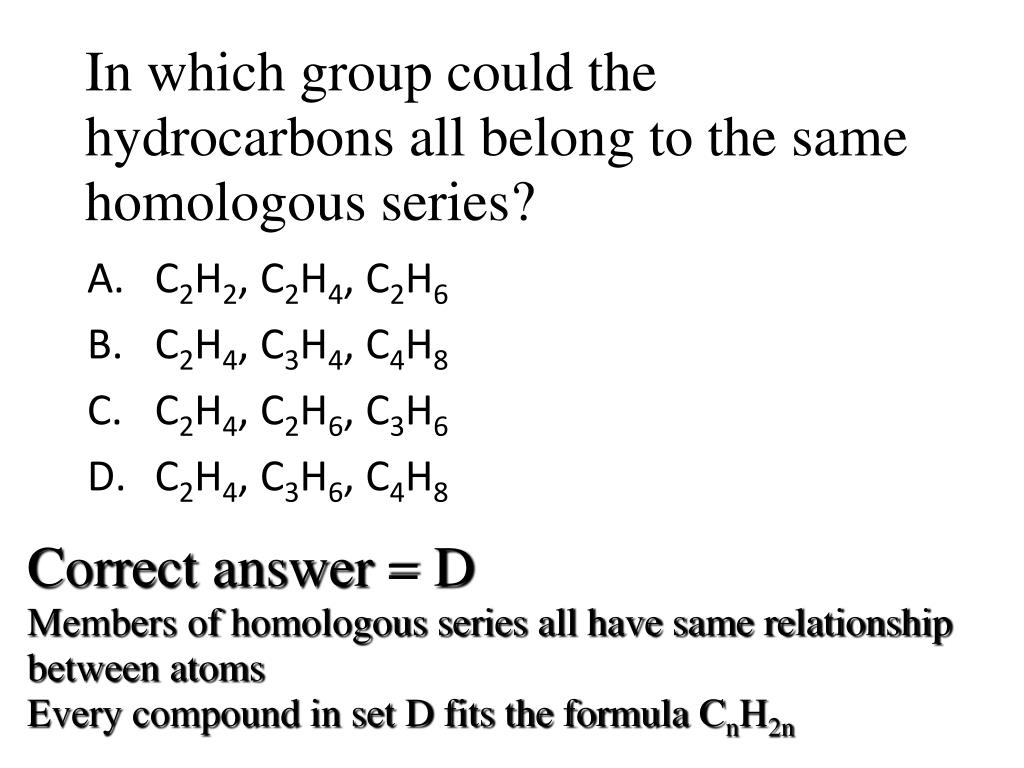
PPT Topic Alkenes Alkynes unsaturated hydrocarbons
Lesson Explainer Homologous Series Nagwa
Structural formula C4H8 having the same molecular formula
Revision PDF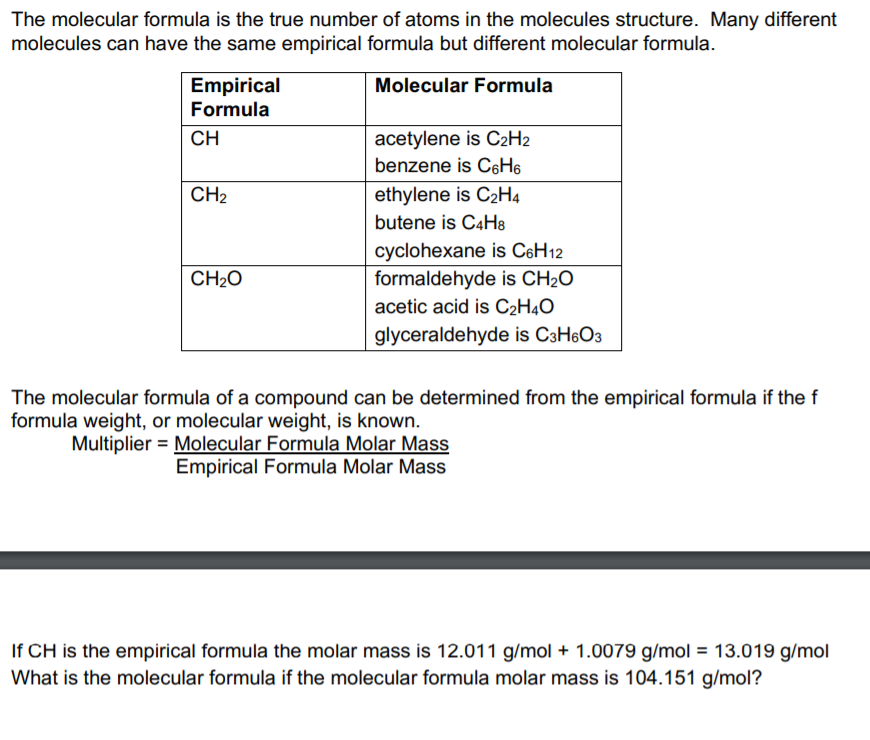
Solved The molecular formula is the true number of atoms in
Recalling the General Formula of a Group of Compounds Using a Displayed Formula
Chemistry Mole Concept Notes LearnPick India
Which sequence represents a portion of a homologous series of
MCAT questions with pictures Flashcards Quizlet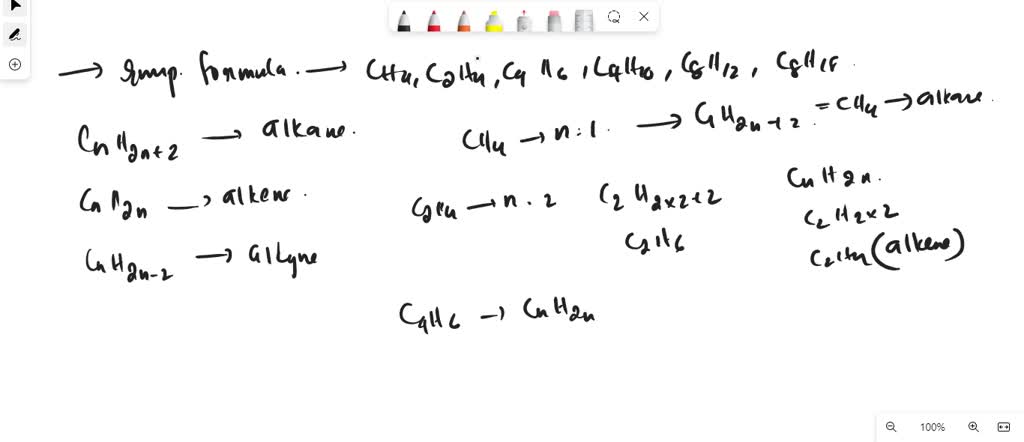
SOLVED 16. Which of the following compounds have the same