Draw Lewis structures for the following molecular formulas C2H4
The correctly drawn lewis hot sale structure for c2h4 will have
Share. Visit »
draw lewis structures for the ethylene molecule c2h4 the
Lewis Dot Structures Neutral Compounds Video Tutorials
How to Draw the Lewis Dot Structure for C2H4 Ethene YouTube
Draw the electron dot structure for C2H4. What is its molecular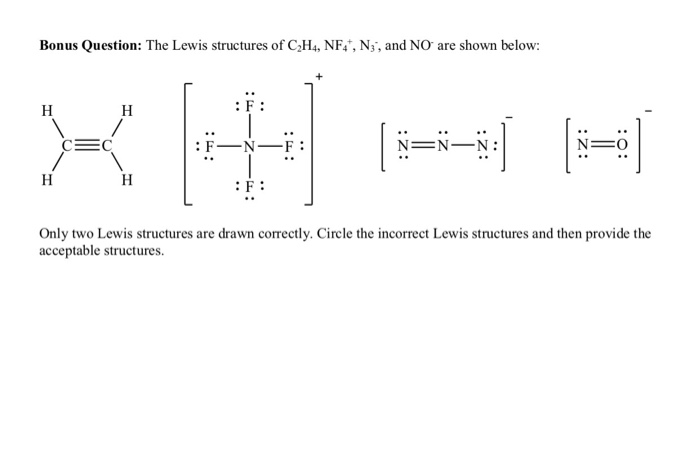
Solved Bonus Question The Lewis structures of C2H4 NF N
Drawing Lewis Dot structures with triple bonds YouTube
Draw the Lewis structures the following molecules and ions
Lewis Symbols and Structures UCalgary Chemistry Textbook
Draw and explain the Lewis structure of C2H4. Homework.Study
DRAW IT Draw Lewis dot structures for each hypothetical molecule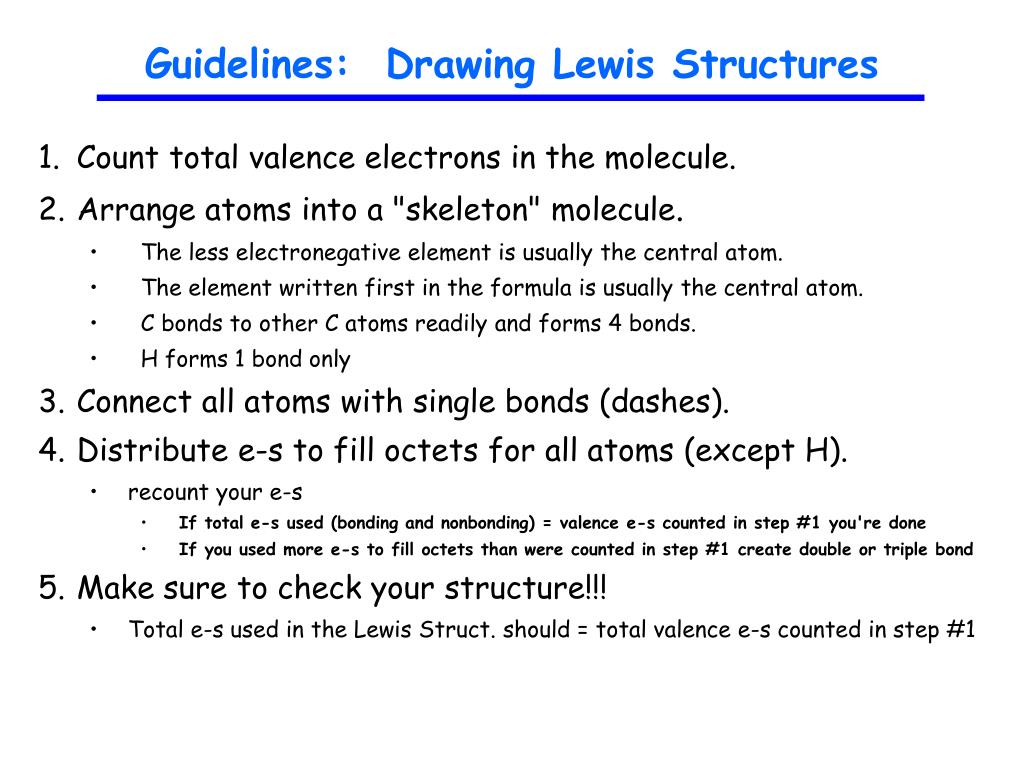
PPT Guidelines Drawing Lewis Structures PowerPoint Presentation
Which is the correct Lewis structure for ethylene C2H4 Quora
9.5 Covalent Bonds and Lewis Structures CHEM 1114 Introduction
C2H4 Lewis Dot Structure How to Draw the Lewis Structure for C2H4
What is the actual lewis structure of C2 Quora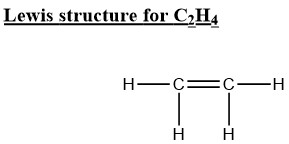
Draw the Lewis structure for ethylene C 2 H 4 . Quizlet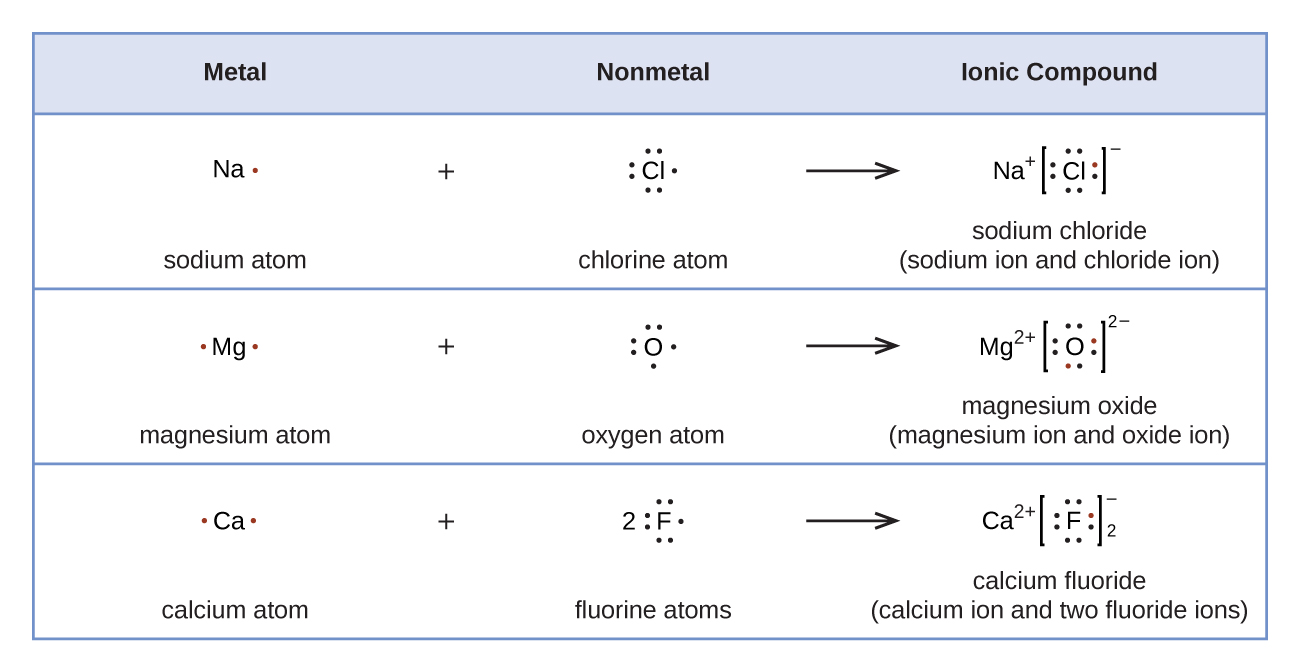
11.3 Lewis Symbols and Structures Enhanced Introductory College
What is the Lewis structure of Ethylene Chemicalbook
Draw the Lewis structure for C2H4 whose skeletal structure is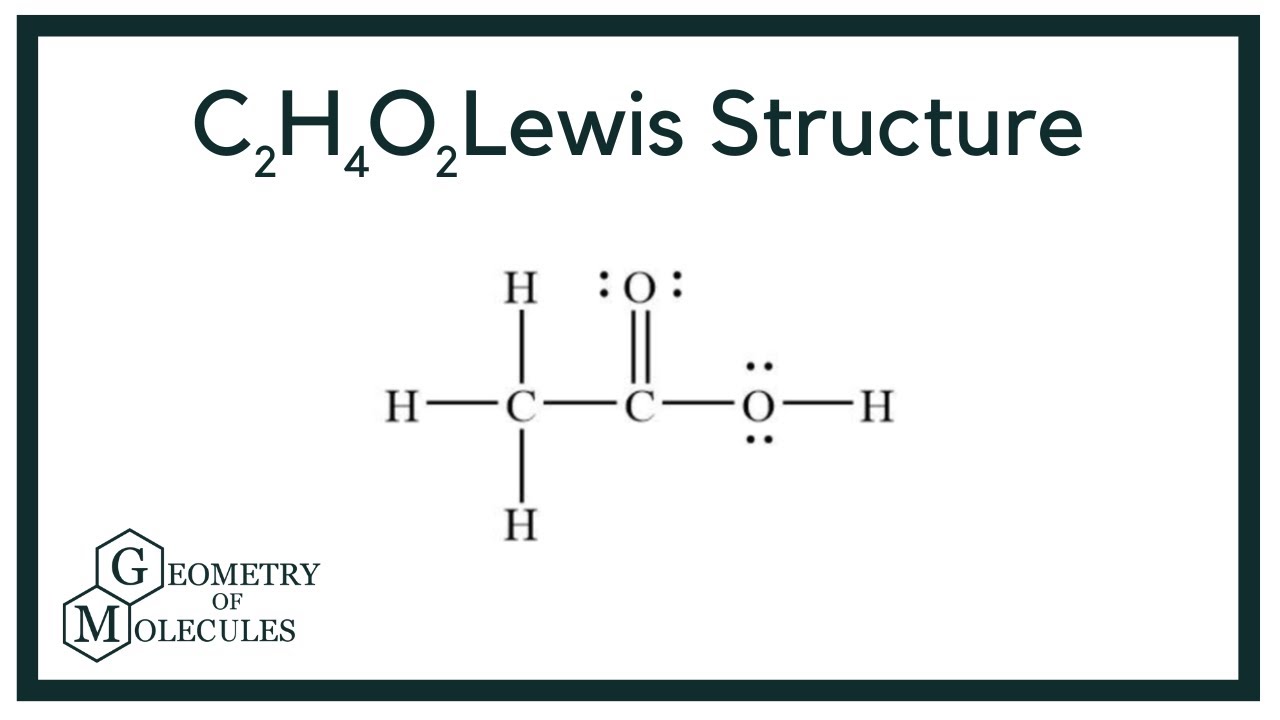
C2H4O2 Lewis structure How to Draw the Lewis Structure for C2H4O2
Draw the molecules. Include all lone pairs of electrons. N2H2 N2H4
C2H4 Lewis Dot Structure How to Draw the Lewis Structure for
Draw the Lewis dot structure for C2H4 and a second structure
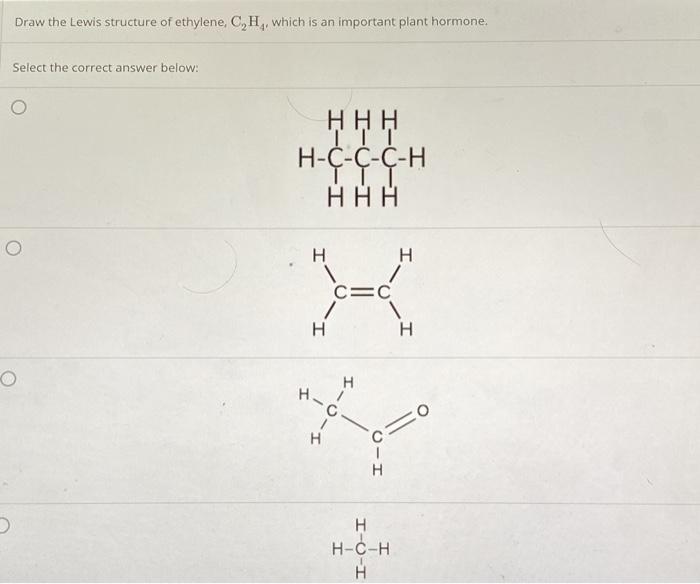
Solved Draw the Lewis structure of ethylene C H. which is
Which is the correct Lewis structure of C2H4 brainly
Lewis Symbols and Structures UCalgary Chemistry Textbook
How is the structural formula for C2H4 determined Quora
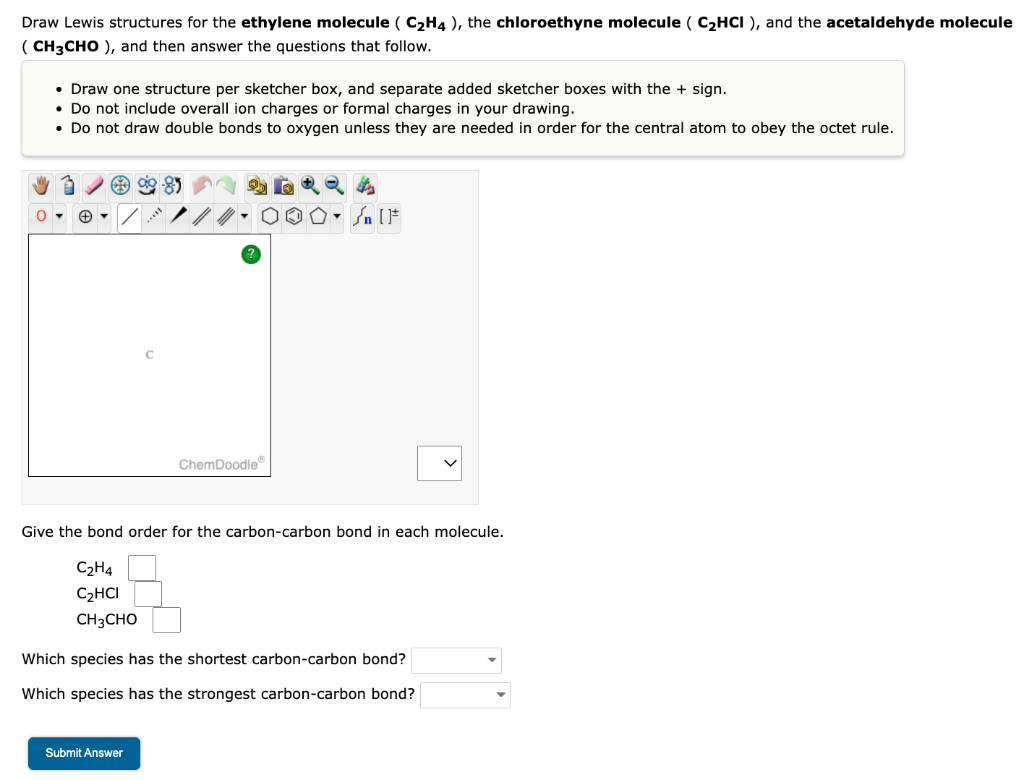
Solved Draw Lewis structures for the ethylene molecule Chegg
10.1 Lewis Structures and the Octet Rule Chemistry LibreTexts
Draw the
Lewis Structure for C2H4
Lewis Structures Simple Organic Compounds Janet Gray Coonce
Draw the electron dot structure of ethene C2H4
Answered Which is the correct Lewis structure bartleby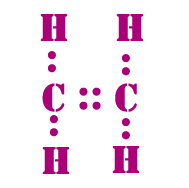
Lewis structure of C2H4 Biochemhelp
How to Draw the Lewis Dot Structure for CH4 Methane
C2H4 Lewis Structure Ethylene
Which is the correct Lewis structure for ethylene C2H4 Quora
C2H4O Lewis Structure Ethylene Oxide