OneClass The combustion of ethane C2H4 occurs via the reaction
Temperature of combustion hot sale c2h4
Share. Visit »
Adiabatic flame temperature and CO concentration of AP HTPB and
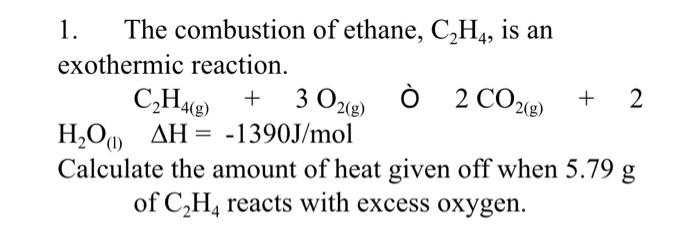
Solved 1. The combustion of ethane C2H4 is an exothermic
heat of combustion of C2H4 is 337 kcal if 5.6 lit o2 is used at
Adiabatic flame temperature versus equivalence ratio for an
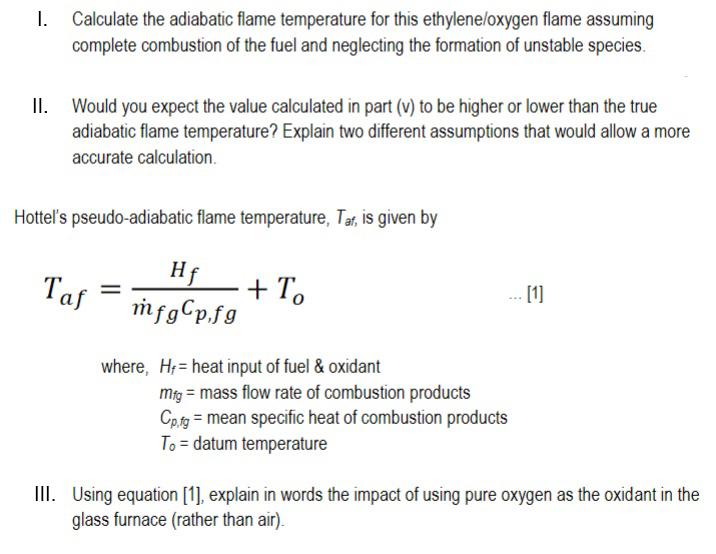
Solved A glass melting furnace is burning ethylene C2H4 in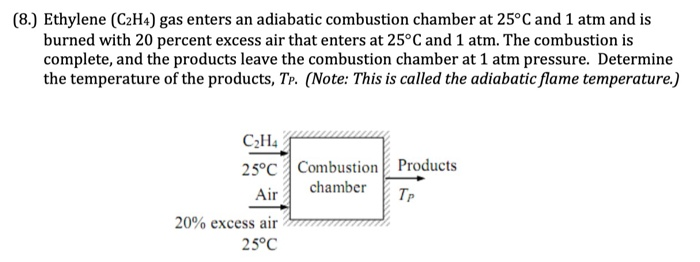
Solved 8. Ethylene C2H4 gas enters an adiabatic Chegg
Answered 5. Write a balanced thermochemical bartleby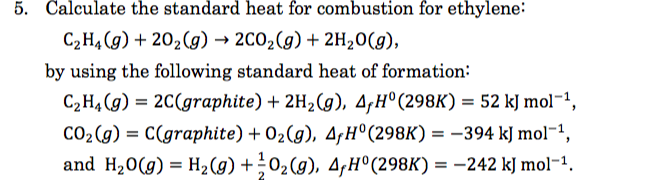
Solved 5 Calculate the standard heat for combustion of Chegg
Answered The combustion of C2H4 g is bartleby
Ethylene Gas Specific Heat vs. Temperature
Be sure to answer all parts. Calculate the heats of combustion for
Ethylene Gas Specific Heat vs. Temperature
39 The heat of combustion of ethene C.H is 1409.3 kJ mol
Heat of combustion of CH4 C2H4 C2H6 are 890 1411 1550 kJ mol
Ethylene Thermophysical Properties
Heat of combustion of CH 4 C 2 H 6 C 2 H 4 and C 2 H 2 gases are 212.8
The heat of combustion of ethylene 17 C and constant volume is
An experimental and modeling study of ethylene air combustion over
Be sure to answer all parts. Calculate the heats of combustion for
An experimental and modeling study of ethylene air combustion over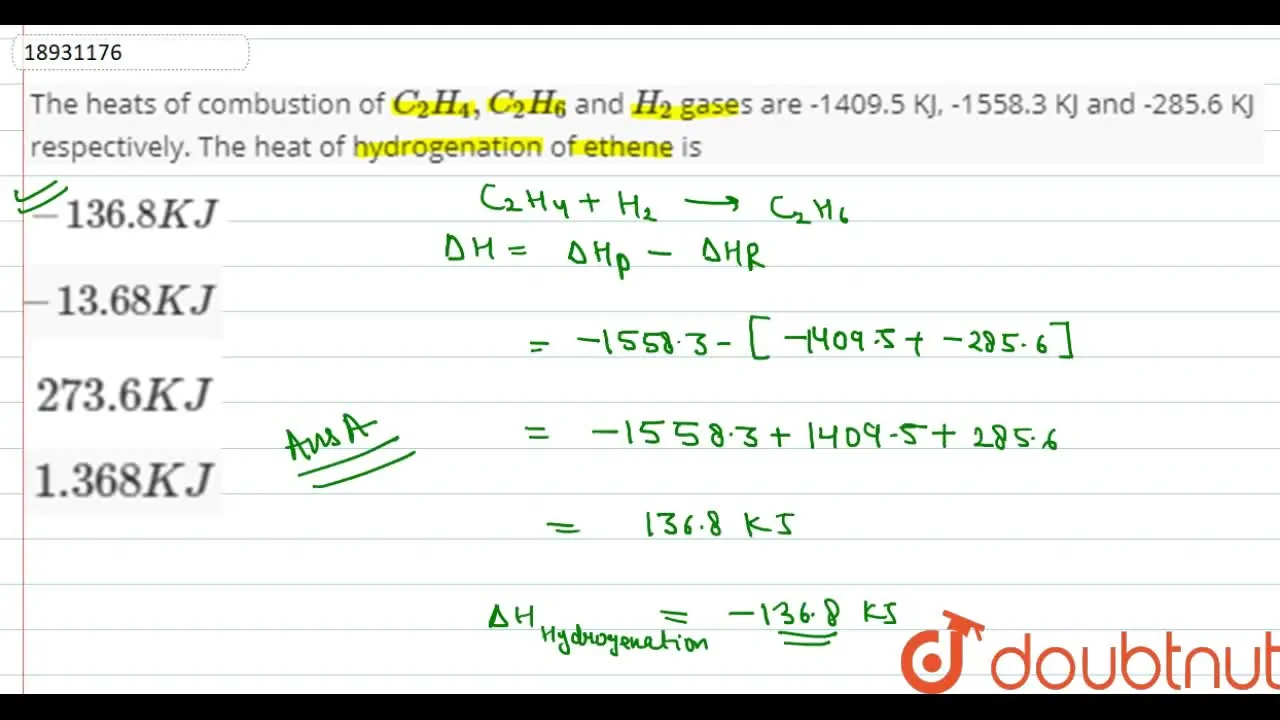
The heats of combustion of C 2 H 4 C 2 H 6 and H 2 gases are 1409
ntif enthalpies of formation for c2h4 g co2 g and h2o l at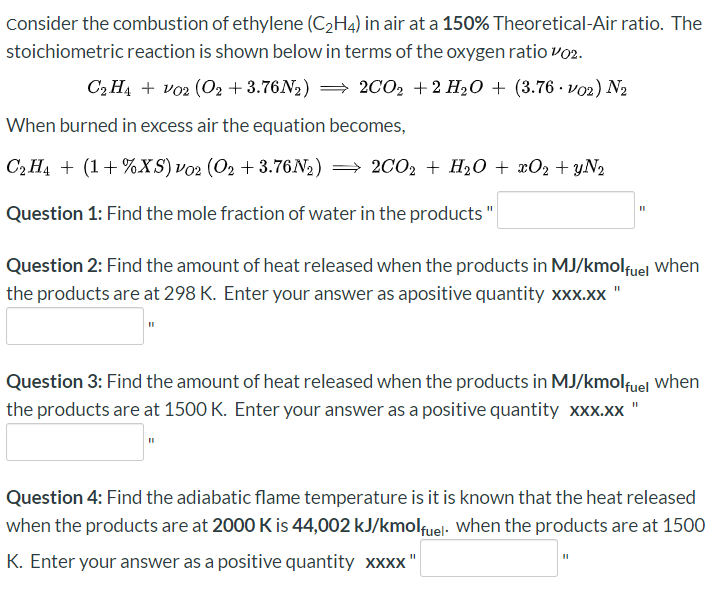
Consider the combustion of ethylene C2H4 in air at Chegg
The heat of combustion of ethylene at 18 C and at constant volume is 335.8 k
42. Standard enthalpies of combustion of C2H4 g C2H6 g and H2 g
Answered For the combustion reaction of ethylene bartleby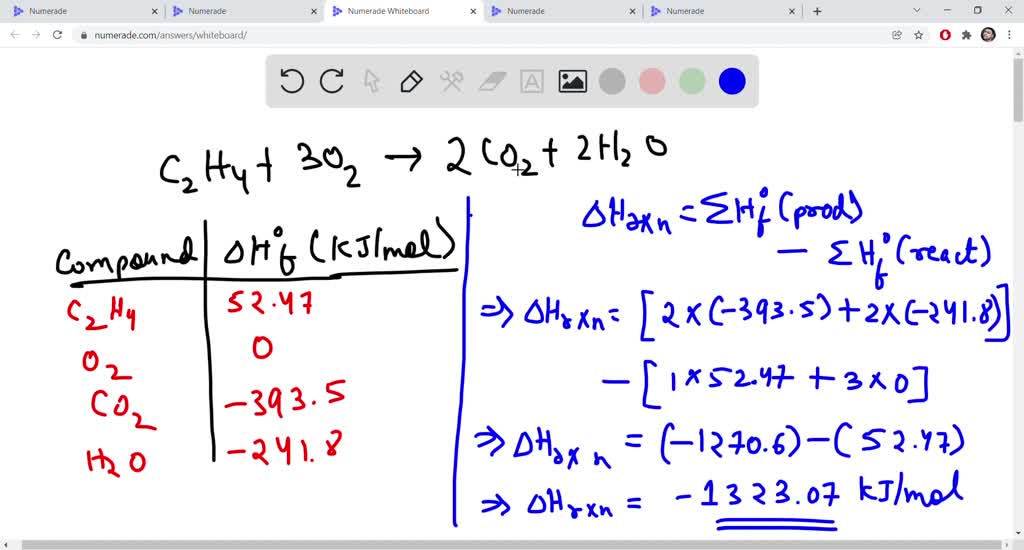
SOLVED The combustion of ethene C2H4 occurs via the reaction
An experimental and modeling study of ethylene air combustion over
Calculate the enthalphy of formation for C2H4. DHf CO2 393.5 kJ mol DHf H2O 285.8 kJ mol
Use bond energies to confirm that the complete combustion of
The thermochemical equation for the combustion of ethylene gas C 2 H 4 is C 2 H 4 g
Calculate the heat of reaction 25 C the reaction C2H4 g H2 g
Calculate the ethalpy of combustion of ethylene g to form CO 2 gas and H 2 O gas at
2 D distributions of temperature T K and soot volume fraction
The heat of combustion of C H C H and H are 1409 kJ mol 1558
Heat of combustion of C 2H 4 is 337 K Cal. If 5.6 lit O 2 is used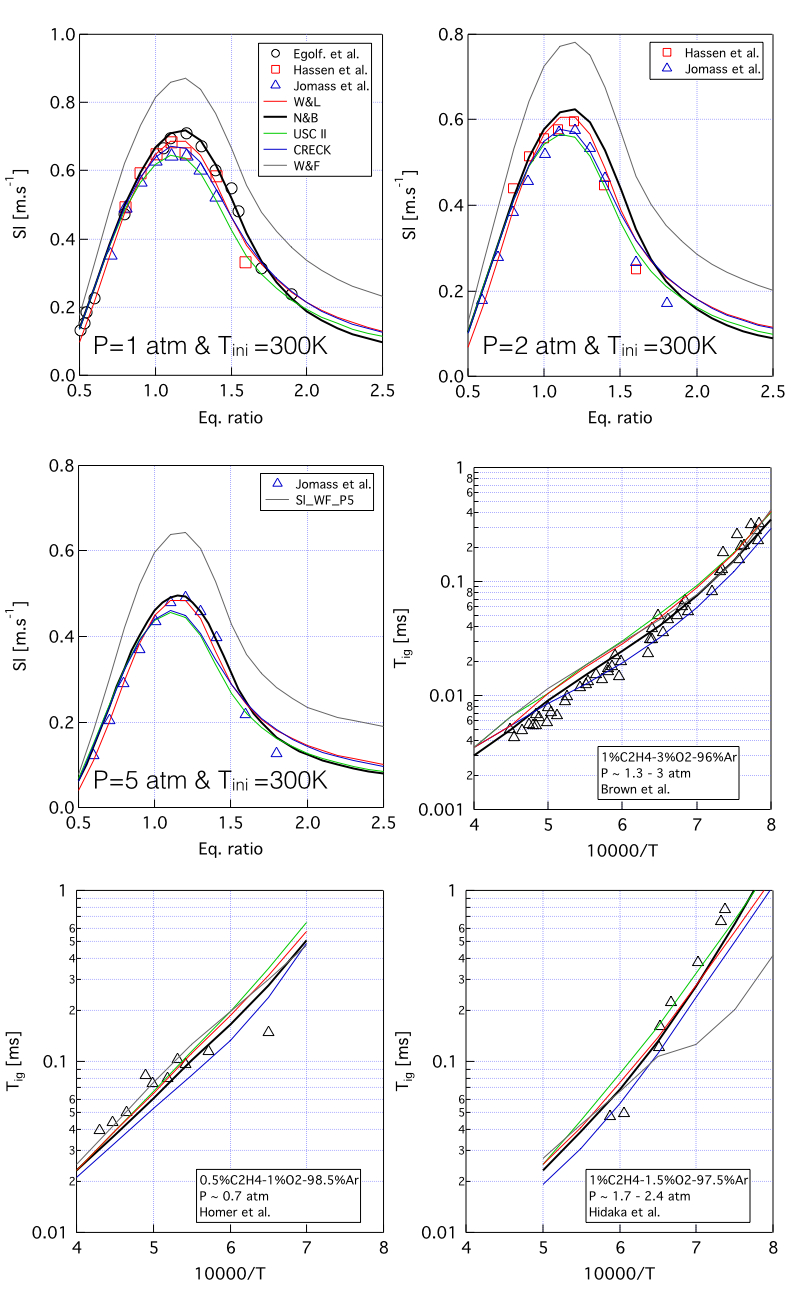
Ethylene air combustion
Uncal 39. The heat of combustion of ethene C H. is 1409.3 kJ
Ethylene C2H4 is burned with 20 excess air in an adiabatic