Answered The standard enthalpy of combustion of bartleby
The combustion of ethene hot sale c2h4 is an exothermic reaction
Share. Visit »
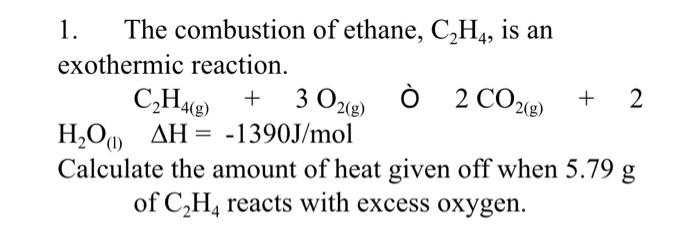
Solved 1. The combustion of ethane C2H4 is an exothermic
If the energy of the reactants in a combustion reaction is 6481 J
OCR A Jun 2017 Paper 1 Q25 with explained solutions
Solved The following equation represents the combustion of Chegg
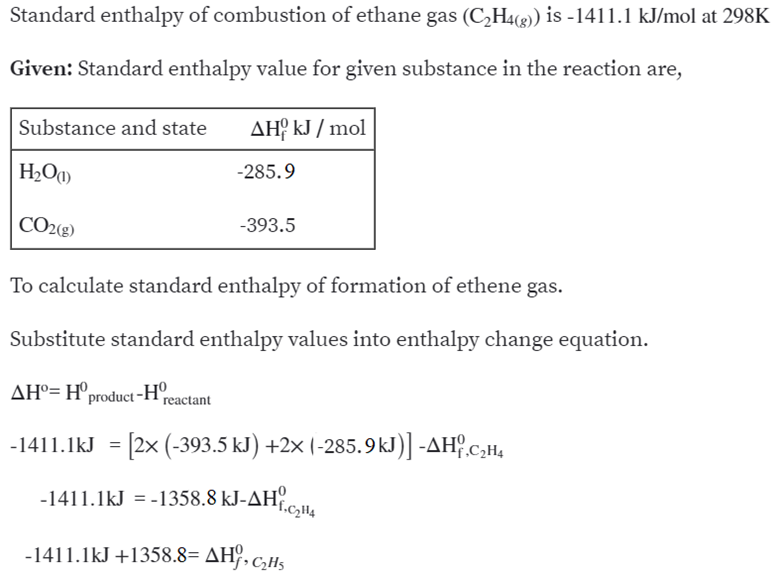
Calculate the enthalphy of formation for C2H4. DHf CO2 393.5 kJ mol DHf H2O 285.8 kJ mol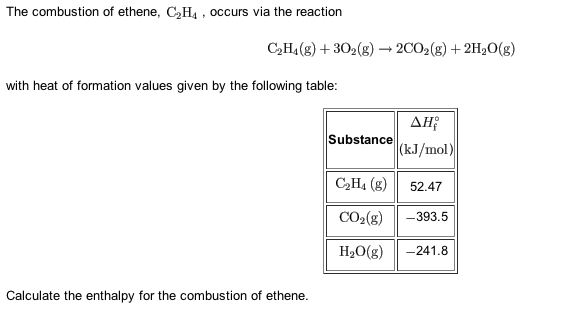
Solved The combustion of ethane C2H4 occurs via the Chegg
Try Yourself 24. Calculate the heat of combustion of ethene CH2
What is the balanced equation when ethane reacts with oxygen Quora
Solved b The enthalpies of combustion for ethene C2H4 Chegg
For complete combustion of ethane C2H4 g 3O2 g 2CO2 g 2H2O l the amount of heat produce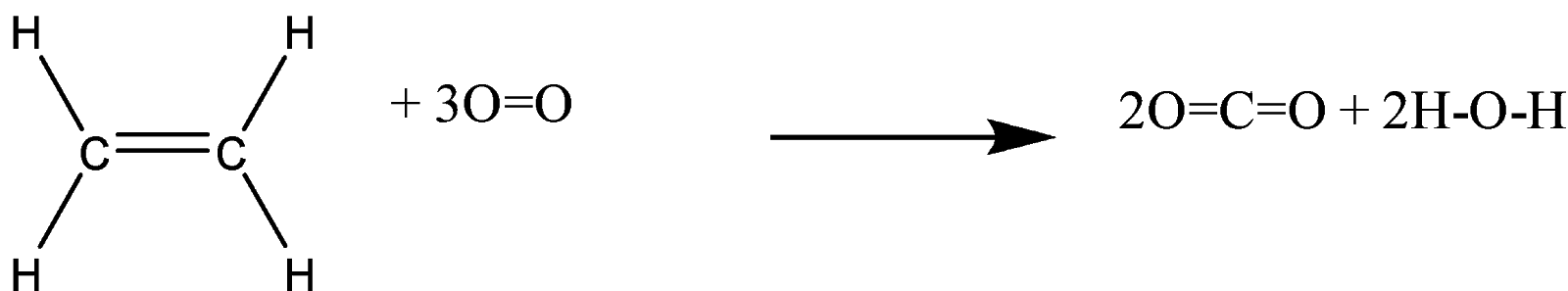
Calculate heat of combustion of ethene from bond energy data C C
Use bond energies to confirm that the complete combustion of
5.7 Enthalpy Calculations Chemistry LibreTexts
Chemistry Thermochemistry 6 of 37 Enthalpy Example 2 Combustion of Ethane
What volume of oxygen required with complete combustion of20dm3
What is the combustion reaction of ethene Quora
Answered 28. The equation below shows the bartleby
Answered The combustion of ethene in the bartleby
Limiting Reactants in Chemistry made SIMPLE chemistry
For complete combustion of ethane C2H4 g 3O2 g 2CO2 g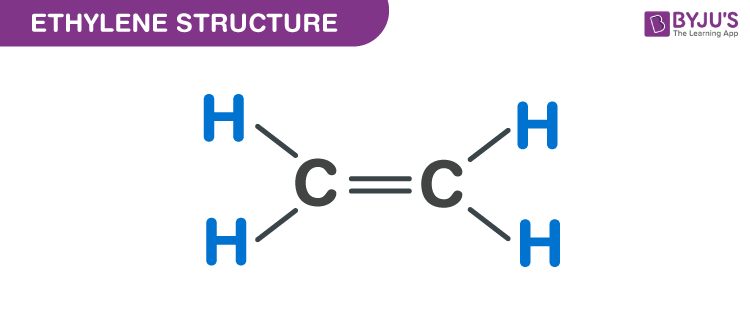
Ethylene C2H4 Structure Molecular Mass Physical and Chemical
Solved Hello I finished almost all the questions I am not 100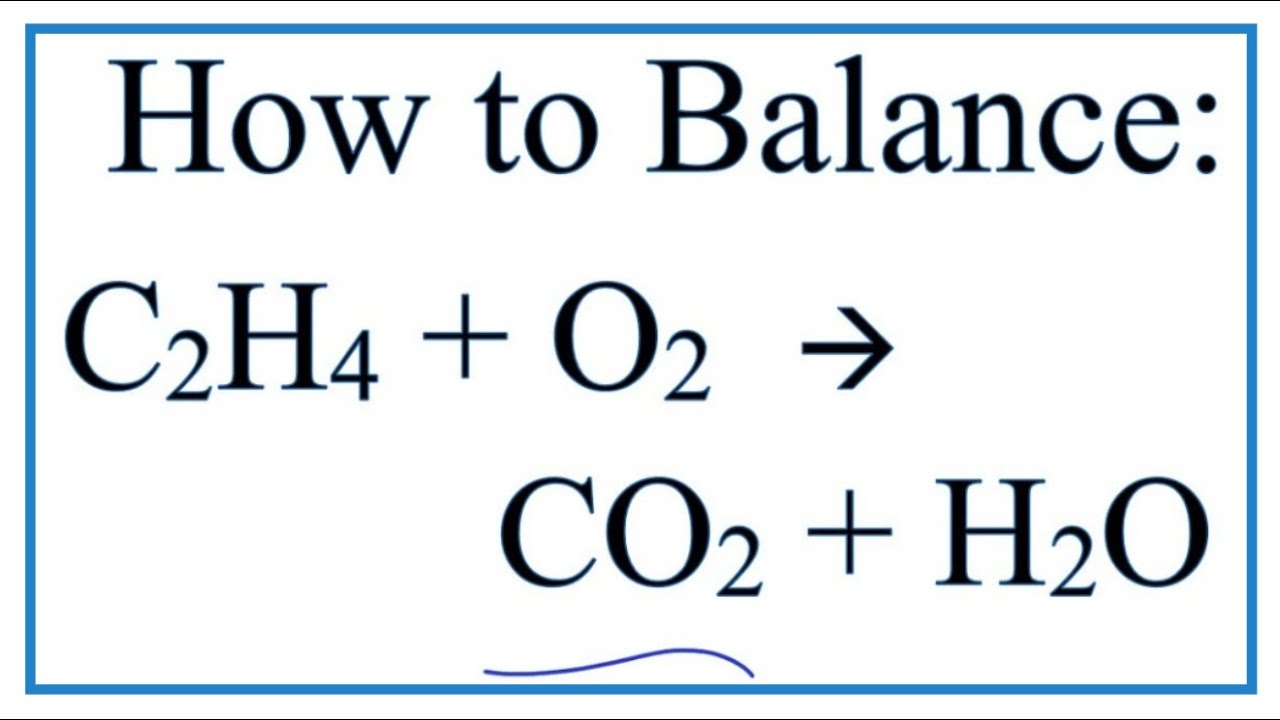
How to Balance C2H4 O2 CO2 H2O Ethene Combustion Reaction
SOLVED The combustion of ethane C2H4 is an exothermic reaction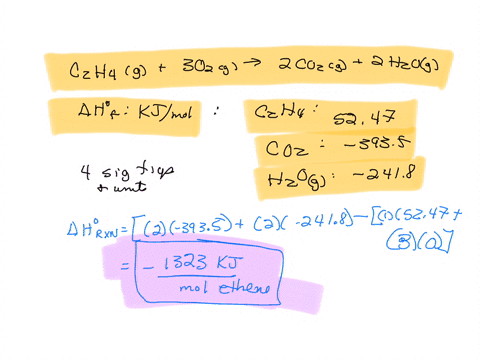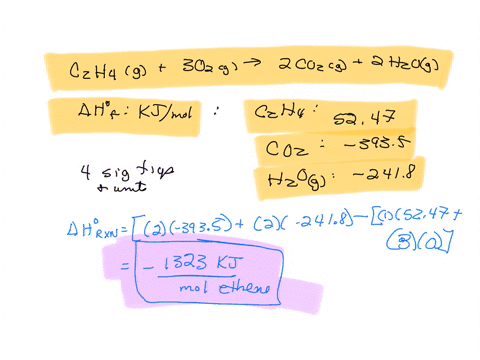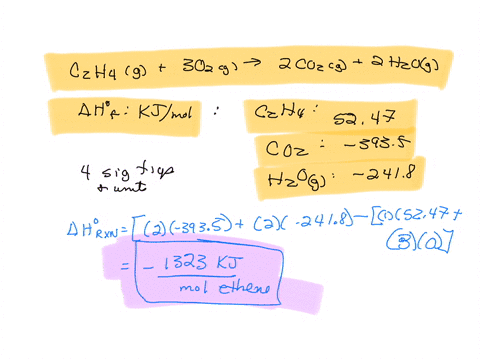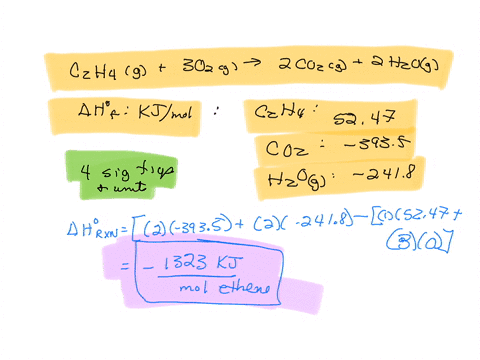
SOLVED The combustion of ethene C2H4 occurs via the reaction
Calculate the heat of reaction 25 C the reaction C2H4 g H2 g
Complete Combustion of Ethane C2H6 Balanced Equation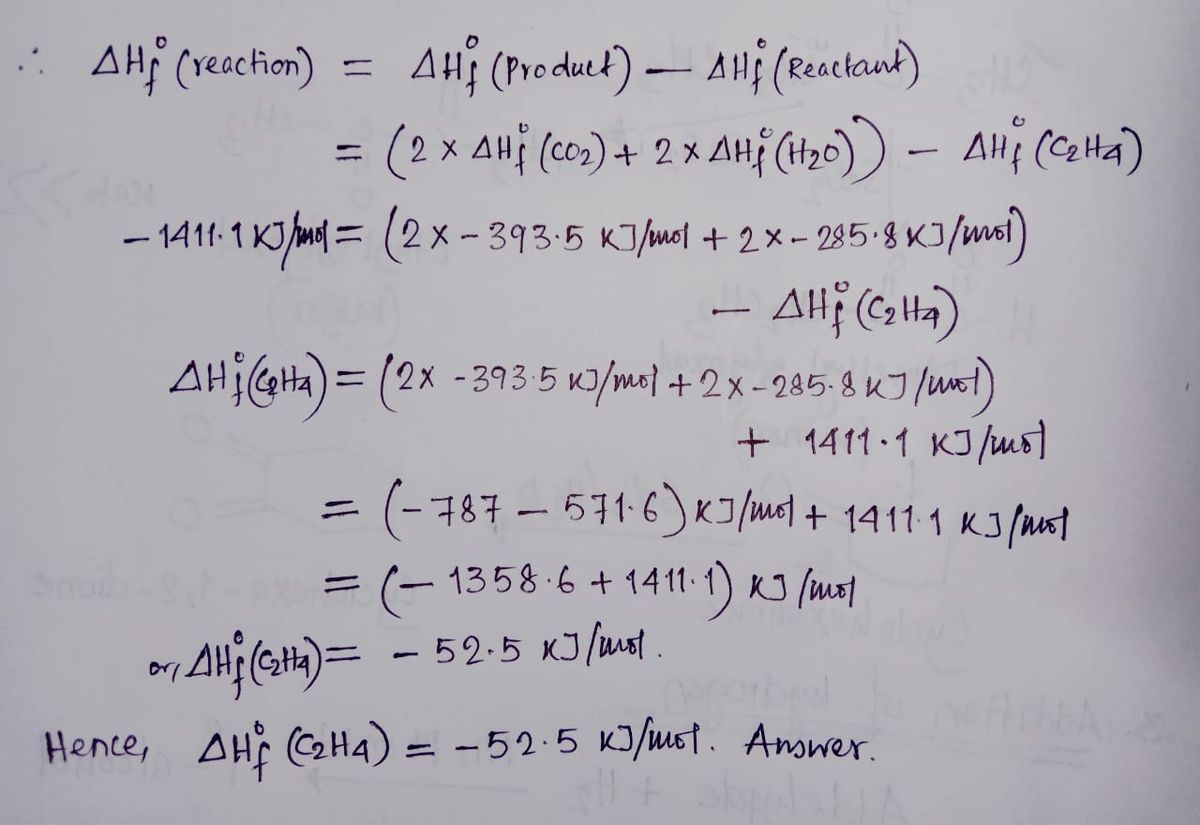
Answered The standard enthalpy of combustion of bartleby
Solved 1. The combustion of ethane C2H4 is an exothermic Chegg
What is the combustion reaction for ethane and balance Quora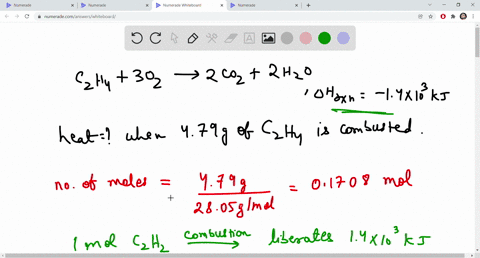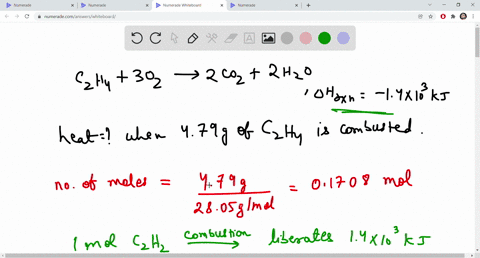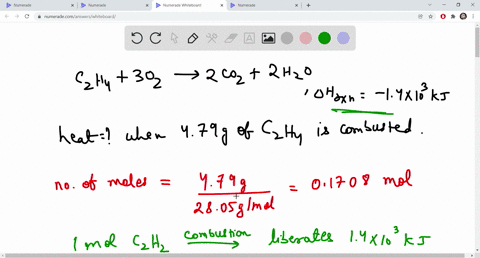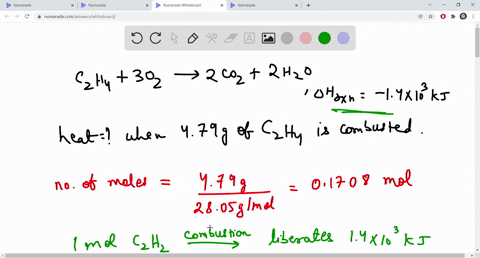
SOLVED The combustion of ethene C2H4 is an exothermic reaction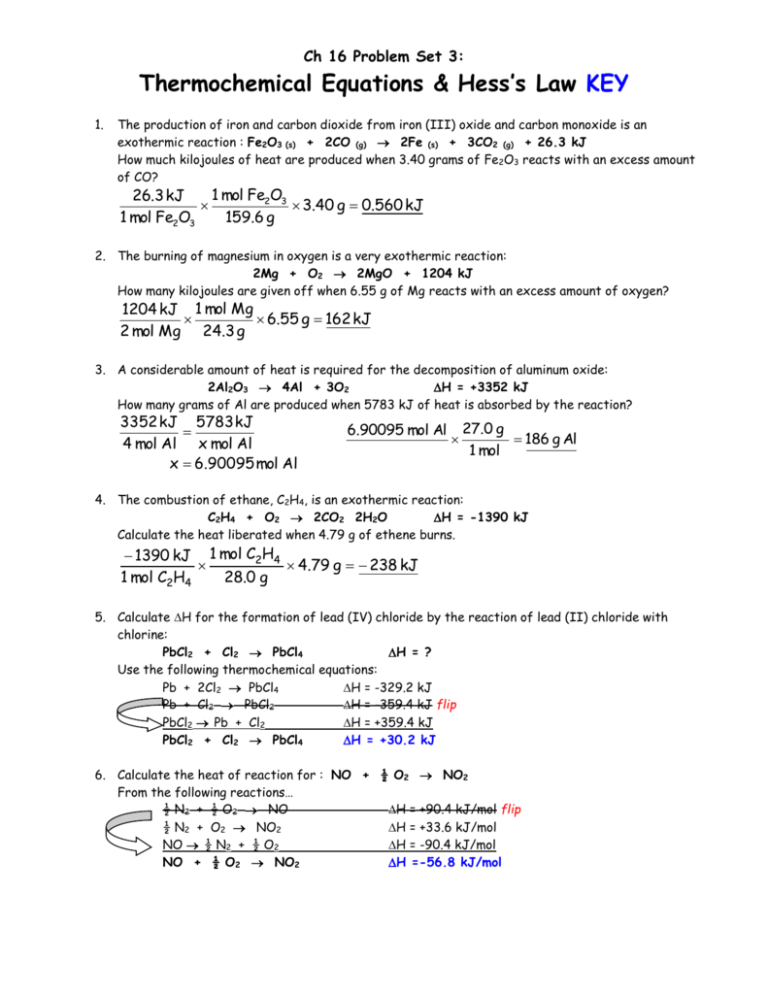
Thermo Eq ns Hess
SOLVED The combustion of ethane C2H4 is an exothermic reaction
Ethene C2H4 can be halogenated by this reaction C2H4 g X2 g
Solved Calculate the enthalpy of reaction for ethene. Assume
Answered 28. The equation below shows the bartleby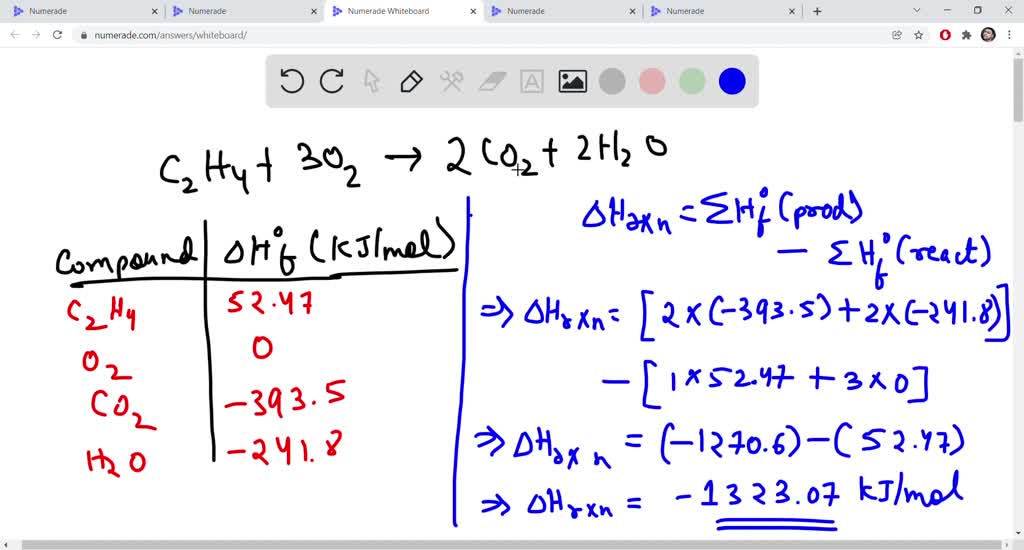
SOLVED The combustion of ethene C2H4 occurs via the reaction
Ethene C2H4 can be halogenated by this reaction C2H4 g X2 g
Use the balanced equation for the combustion of ethane to complete